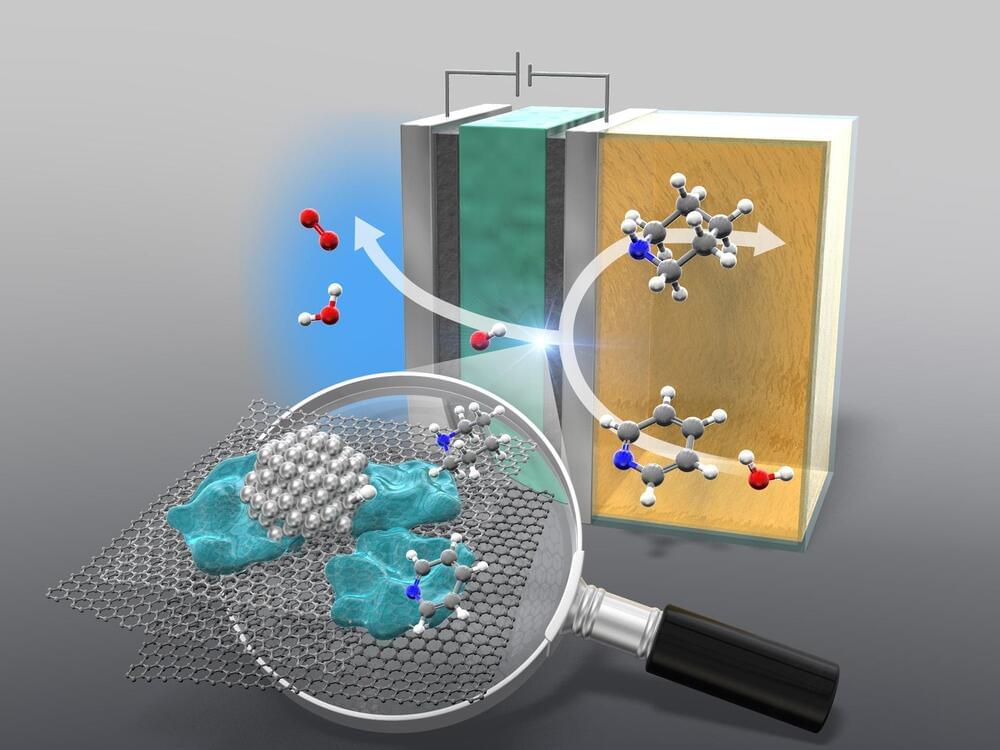
A new study introduces an eco-friendly method using an AEM electrolyzer to hydrogenate cyclic amines, reducing the chemical industry’s carbon emissions. This process replaces fossil fuels with water and renewable electricity, maintaining high efficiency.
To reduce the environmental impact of the chemical manufacturing industry, it is crucial to develop greener methods for producing the chemical building blocks of widely used compounds.
It’s no secret manufacturing processes have some of the most impactful and intense effects on the environment, with the chemical manufacturing industry topping the charts for both energy consumption and emissions output. While this makes sense thanks to the grand scale in which manufactured chemicals are involved in daily life, it still leaves a lot to be desired for sustainability’s sake. By focusing on renewable energy sources and alternative methods for creating the chemical building blocks of some of the most commonly used compounds, researchers hope to reduce the chemical manufacturing industry’s footprint with some green innovation.