Researchers have made a significant advance toward the goal of using bacteria to produce ethylene, a key manufacturing chemical.


For the new study, the researchers used samples from the brains of normal mice as well as living cortical brain tissue sampled with permission from six individuals undergoing surgical treatment for epilepsy. The surgical procedures were medically necessary to remove lesions from the brain’s hippocampus.
The researchers first validated the zap-and-freeze approach by observing calcium signaling, a process that triggers neurons to release neurotransmitters in living mouse brain tissues.
Next, the scientists stimulated neurons in mouse brain tissue with the zap-and-freeze approach and observed where synaptic vesicles fuse with brain cell membranes and then release chemicals called neurotransmitters that reach other brain cells. The scientists then observed how mouse brain cells recycle synaptic vesicles after they are used for neuronal communication, a process known as endocytosis that allows material to be taken up by neurons.
The researchers then applied the zap-and-freeze technique to brain tissue samples from people with epilepsy, and observed the same synaptic vesicle recycling pathway operating in human neurons.
In both mouse and human brain samples, the protein Dynamin1xA, which is essential for ultrafast synaptic membrane recycling, was present where endocytosis is thought to occur on the membrane of the synapse.
“Our findings indicate that the molecular mechanism of ultrafast endocytosis is conserved between mice and human brain tissues,” the author says, suggesting that the investigations in these models are valuable for understanding human biology.
Quantum computers will need large numbers of qubits to tackle challenging problems in physics, chemistry, and beyond. Unlike classical bits, qubits can exist in two states at once—a phenomenon called superposition. This quirk of quantum physics gives quantum computers the potential to perform certain complex calculations better than their classical counterparts, but it also means the qubits are fragile. To compensate, researchers are building quantum computers with extra, redundant qubits to correct any errors. That is why robust quantum computers will require hundreds of thousands of qubits.
Now, in a step toward this vision, Caltech physicists have created the largest qubit array ever assembled: 6,100 neutral-atom qubits trapped in a grid by lasers. Previous arrays of this kind contained only hundreds of qubits.
This milestone comes amid a rapidly growing race to scale up quantum computers. There are several approaches in development, including those based on superconducting circuits, trapped ions, and neutral atoms, as used in the new study.
The neutral-atom platform shows promise for scaling up quantum computers.
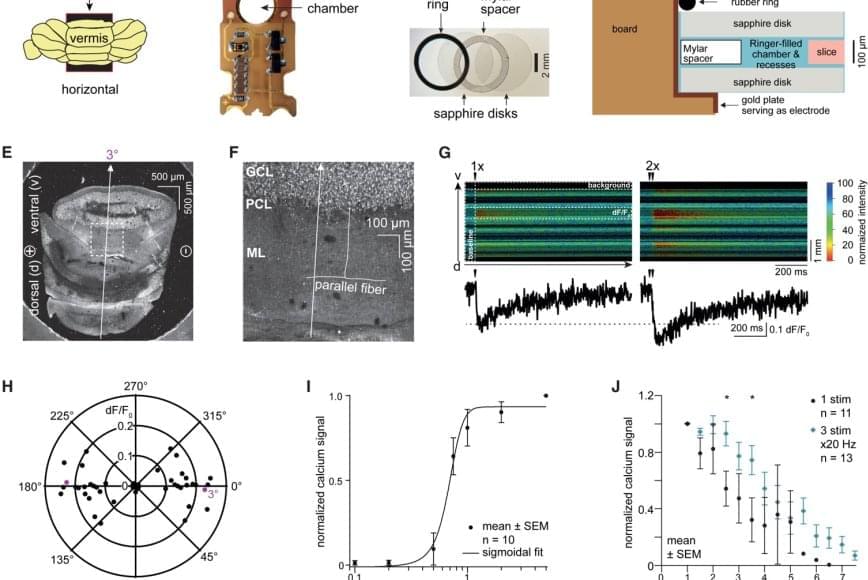
Boron, a chemical element next to carbon in the periodic table, is known for its unique ability to form complex bond networks. Unlike carbon, which typically bonds with two or three neighboring atoms, boron can share electrons among several atoms. This leads to a wide variety of nanostructures. These include boron fullerenes, which are hollow, cage-like molecules, and borophenes, ultra-thin metallic sheets of boron atoms arranged in triangular and hexagonal patterns.
Dr. Nevill Gonzalez Szwacki has developed a model explaining the variety of boron nanostructures. The analysis, published in the journal 2D Materials, combines more than a dozen known boron nanostructures, including the experimentally observed B₄₀ and B₈₀ fullerenes.
Using first-principles quantum-mechanical calculations, the study shows that the structural, energetic, and electronic properties of these systems can be predicted by looking at the proportions of atoms with four, five, or six bonds. The results reveal clear links between finite and extended boron structures. The B₄₀ cage corresponds to the χ₃ borophene layer, while B₆₅, B₈₀, and B₉₂ connect with the β₁₂, α, and bt borophene sheets, respectively. These structural links suggest that new boron cages could be created by using known two-dimensional boron templates.
Leading synthetic biologists have shared hard-won lessons from their decade-long quest to build the world’s first synthetic eukaryotic genome in a Nature Biotechnology paper. Their insights could accelerate development of the next generation of engineered organisms, from climate-resilient crops to custom-built cell factories.
“We’ve assembled a comprehensive overview of the literature on how to build a lifeform where we review what went right—but also what went wrong,” says Dr. Paige Erpf, lead author of the paper and postdoctoral researcher at Macquarie University’s School of Natural Sciences and the Australian Research Council (ARC) Center of Excellence in Synthetic Biology.
The Synthetic Yeast Genome Project (Sc2.0) involved a large, evolving global consortium of 200-plus researchers from more than ten institutions, who jointly set out to redesign and chemically synthesize all 16 chromosomes of baker’s yeast from scratch. Macquarie University contributed to the synthesis of two of these chromosomes, comprising around 12% of the project overall.
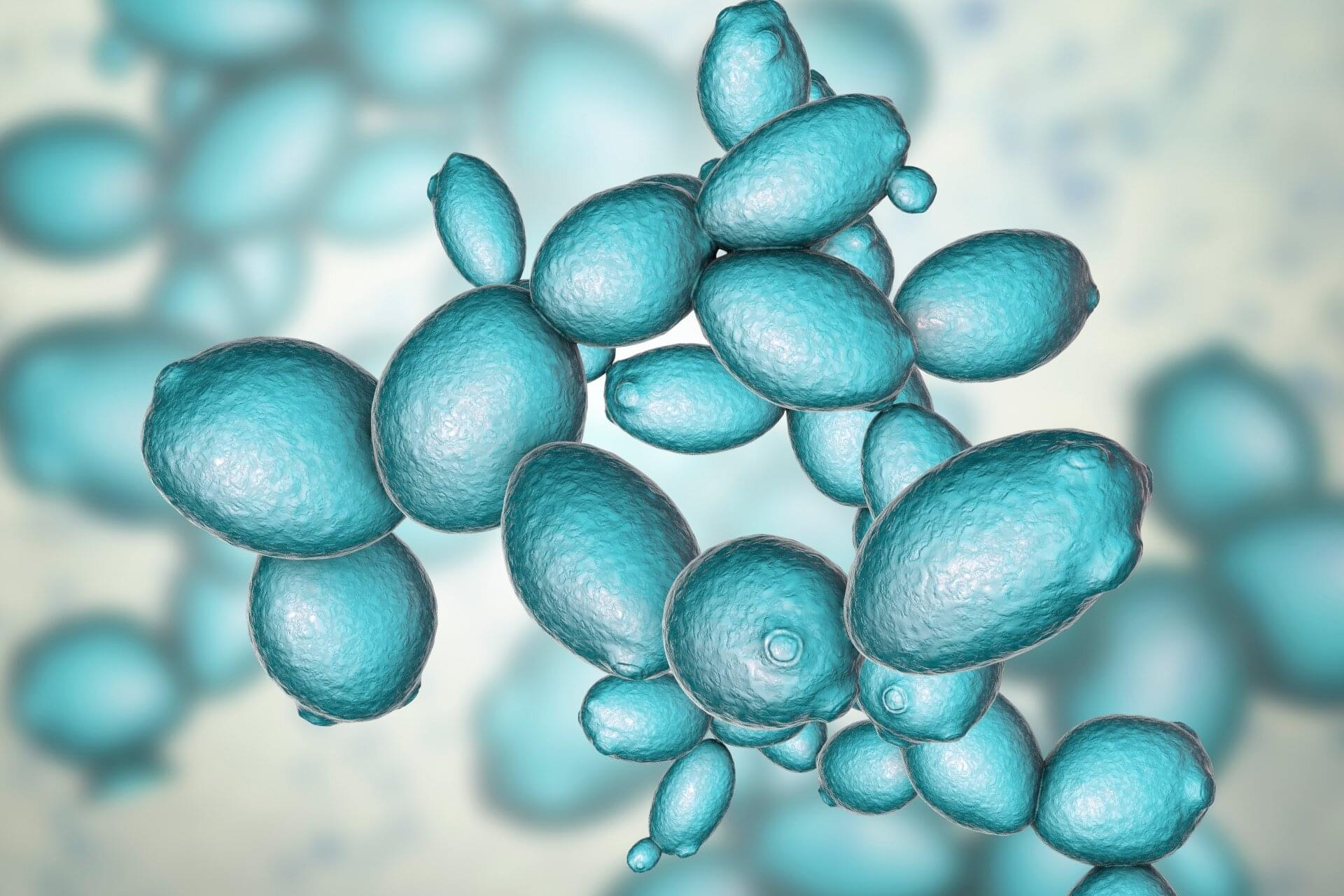
In a study published in Journal of the American Chemical Society, a team led by Prof. Song Li from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences synthesized monolayer WS2 lateral homojunctions via in situ domain engineering, and enabled controllable direct chemical vapor deposition (CVD) growth of these structures.
Two-dimensional (2D) transition metal dichalcogenides are ideal candidates to replace silicon-based semiconductors due to their exceptional electrical properties at atomic scales. However, device applications require heterogeneous field-effect modulation behaviors across low-dimensional units. Van der Waals interactions or lateral atomic bonding allow damage-free integration into homojunctions/heterojunctions, but direct epitaxy growth remains challenging due to strict atomic species constraints.
In this study, researchers first determined optimal intrinsic defect configurations through theoretical simulations. Then they employed a two-step CVD method to achieve the in situ modulation of defect structures at the domain level, yielding homojunctions with tailored defect architectures.
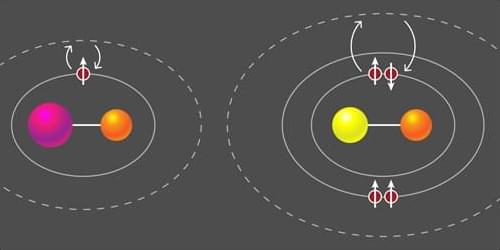
A class of molecules with two valence electrons has been laser cooled and trapped for the first time.
Over the past 70 years, physicists have developed laser-based methods for controlling atoms and molecules, but much of this success has been concentrated on a few columns of the periodic table. For molecules, laser cooling has been limited to diatomic species that have a single unpaired valence electron for interacting with light. Extending laser cooling to molecules with two valence electrons has long been sought after (Fig. 1). The most promising nonreactive candidates are diatomic molecules that partner a halogen, such as fluorine (F) or chlorine (Cl), with a p-block atom, such as aluminum (Al) or thallium (Tl). Several research groups have specifically targeted AlF, AlCl, and TlF, but these molecules are difficult to work with because of their deep-ultraviolet transitions, complicated energy-level structures, and small magnetic moments.
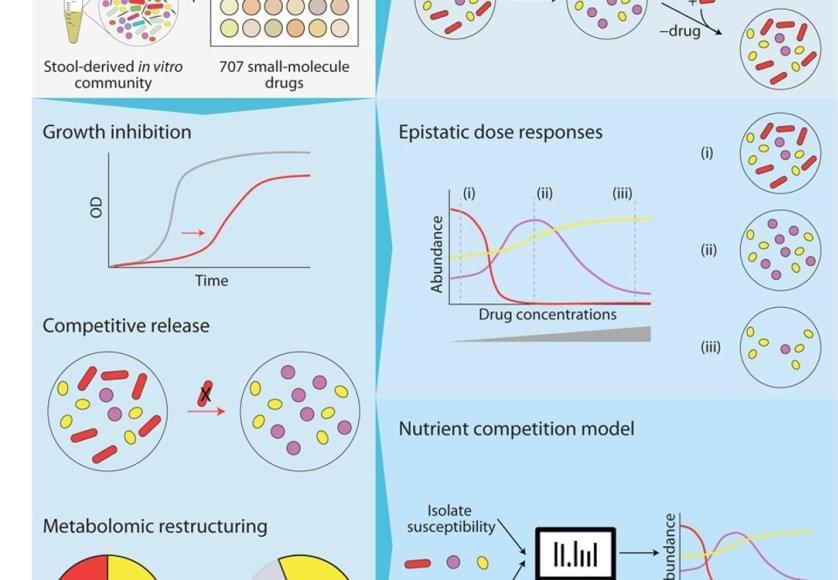
The bacteria in our poop are a reasonable representation of what’s living in our digestive system. To understand how different drugs can impact the gut microbiome, the team cultured microbial communities from nine donor fecal samples and systematically tested them with 707 different clinically relevant drugs.
The researchers examined changes in the growth of different bacterial species, the community composition, and the metabolome – the mix of small molecules called metabolites that microbes produce and consume. They found that 141 drugs altered the microbiome of the samples and even short-term treatments created enduring changes, entirely wiping out some microbial species. The primary force behind how the community responds to drug inhibition was competition over nutrients.
“The winners and losers among our gut bacteria can often be predicted by understanding how sensitive they are to the medications and how they compete for food,” said the first author on the paper. “In other words, drugs don’t just kill bacteria; they also reshuffle the ‘buffet’ in our gut, and that reshuffling shapes which bacteria win.”
Despite the complexity of the bacterial communities, the researchers were able to create data-driven computer models that accurately predicted how they would respond to a particular drug. They factored in the sensitivity of different bacterial species to that drug and the competitive landscape – essentially, who was competing with whom for which nutrients.
Their work provides a framework for predicting how a person’s microbial community might change with a given drug, and could help scientists find ways to prevent these changes or more easily restore a healthy gut microbiome in the future.
Our gut microbiome is made up of trillions of bacteria and other microbes living in our intestines. These help our bodies break down food, assist our immune system, send chemical signals to our brain, and potentially serve many other functions that researchers are still working to understand. When the microbiome is out of balance – with not enough helpful bacteria or the wrong combination of microbes – it can affect our whole body.
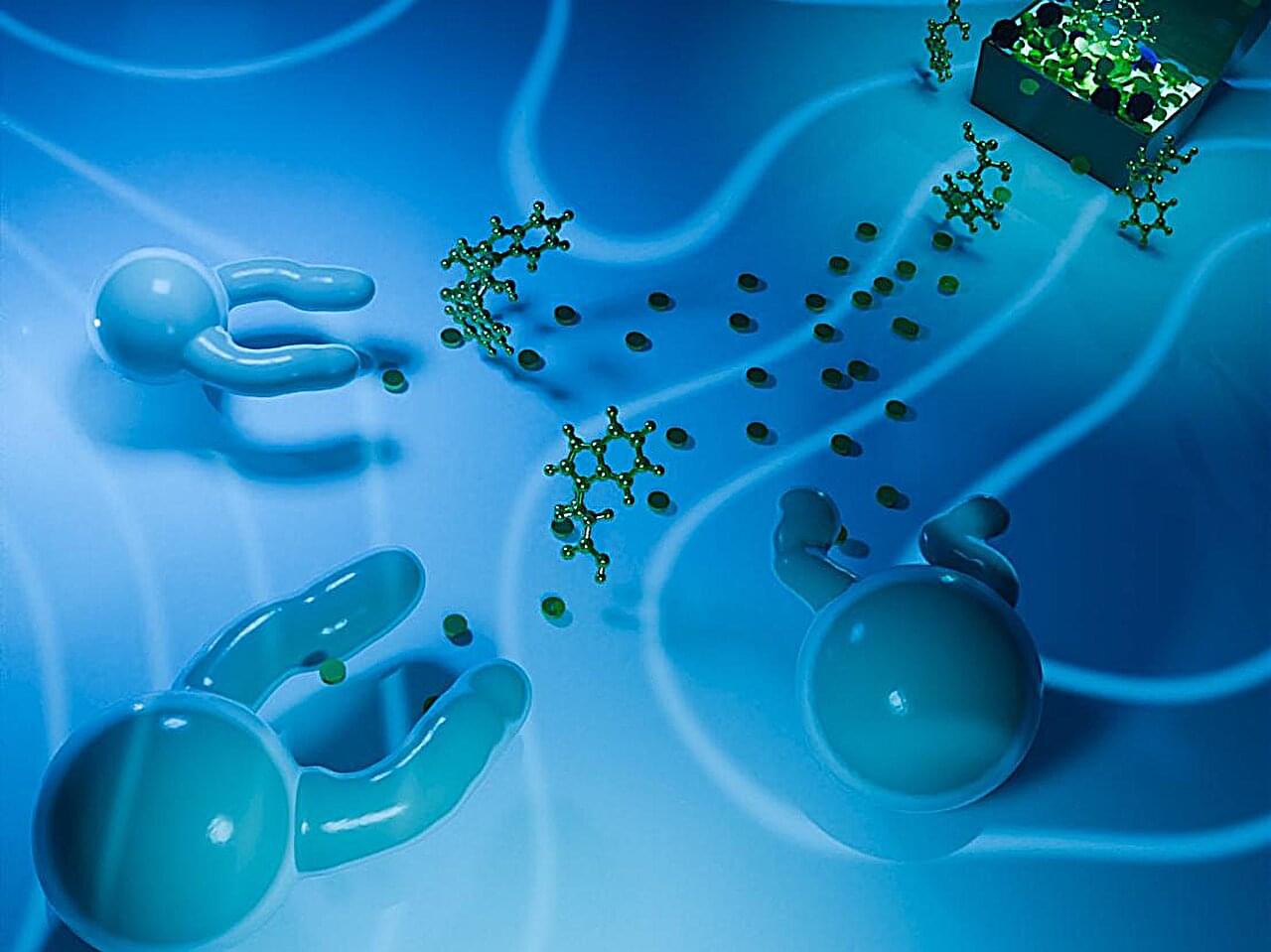
Oil-in-water droplets respond to chemical cues by forming arm-like extensions that resemble filopodia, which are used by living cells to sense and explore their environment.
A research team led by chemists at Penn State studies the droplets to glimpse how matter may have transitioned to life billions of years ago. The researchers have dissected the mechanism through which these arms form and shown that they respond directionally, growing toward or away from specific chemicals.
The research appears in the Journal of the American Chemical Society and will be featured on the front cover of an upcoming issue. The society also featured the research in its Research Headlines video series, which spotlights new and interesting work published in the society’s journals.