A decades-old challenge in neuroscience has been solved by harnessing artificial intelligence (AI) to identify the electrical signatures of different types of brain cells for the first time, as part of a study in mice led by researchers from UCL.



What if your conscious experiences were not just the chatter of neurons, but were connected to the hum of the universe? In a paper published in Frontiers in Human Neuroscience, I present new evidence indicating that conscious states may arise from the brain’s capacity to resonate with the quantum vacuum—the zero-point field that permeates all of space.
More specifically, I argue that macroscopic quantum effects are at play inside our heads. This insight results from a synthesis of brain architectural and neurophysiological findings supplemented with quantitative model calculations. The novel synthesis suggests that the brain’s basic functional building blocks, cortical microcolumns, couple directly to the zero-point field, igniting the complex dynamics characteristic of conscious processes.

An emerging body of evidence indicates that short-term immersion in cold water facilitates positive affect and reduces negative affect. However, the neural mechanisms underlying these effects remain largely unknown. For the first time, we employed functional magnetic resonance imaging (fMRI) to identify topological clusters of networks coupled with behavioural changes in positive and negative affect after a 5 min cold-water immersion. Perceived changes in positive affect were associated with feeling more active, alert, attentive, proud, and inspired, whilst changes in negative affect reflected reductions in distress and nervousness.
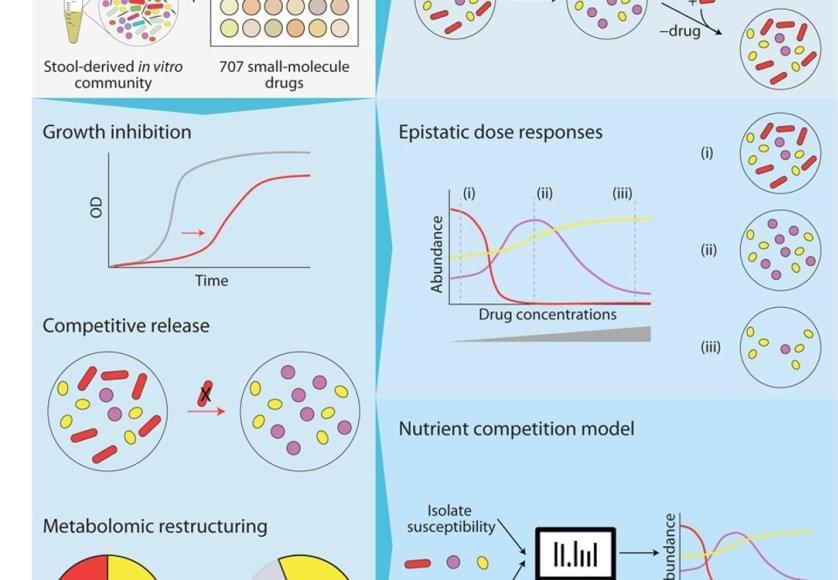
The bacteria in our poop are a reasonable representation of what’s living in our digestive system. To understand how different drugs can impact the gut microbiome, the team cultured microbial communities from nine donor fecal samples and systematically tested them with 707 different clinically relevant drugs.
The researchers examined changes in the growth of different bacterial species, the community composition, and the metabolome – the mix of small molecules called metabolites that microbes produce and consume. They found that 141 drugs altered the microbiome of the samples and even short-term treatments created enduring changes, entirely wiping out some microbial species. The primary force behind how the community responds to drug inhibition was competition over nutrients.
“The winners and losers among our gut bacteria can often be predicted by understanding how sensitive they are to the medications and how they compete for food,” said the first author on the paper. “In other words, drugs don’t just kill bacteria; they also reshuffle the ‘buffet’ in our gut, and that reshuffling shapes which bacteria win.”
Despite the complexity of the bacterial communities, the researchers were able to create data-driven computer models that accurately predicted how they would respond to a particular drug. They factored in the sensitivity of different bacterial species to that drug and the competitive landscape – essentially, who was competing with whom for which nutrients.
Their work provides a framework for predicting how a person’s microbial community might change with a given drug, and could help scientists find ways to prevent these changes or more easily restore a healthy gut microbiome in the future.
Our gut microbiome is made up of trillions of bacteria and other microbes living in our intestines. These help our bodies break down food, assist our immune system, send chemical signals to our brain, and potentially serve many other functions that researchers are still working to understand. When the microbiome is out of balance – with not enough helpful bacteria or the wrong combination of microbes – it can affect our whole body.
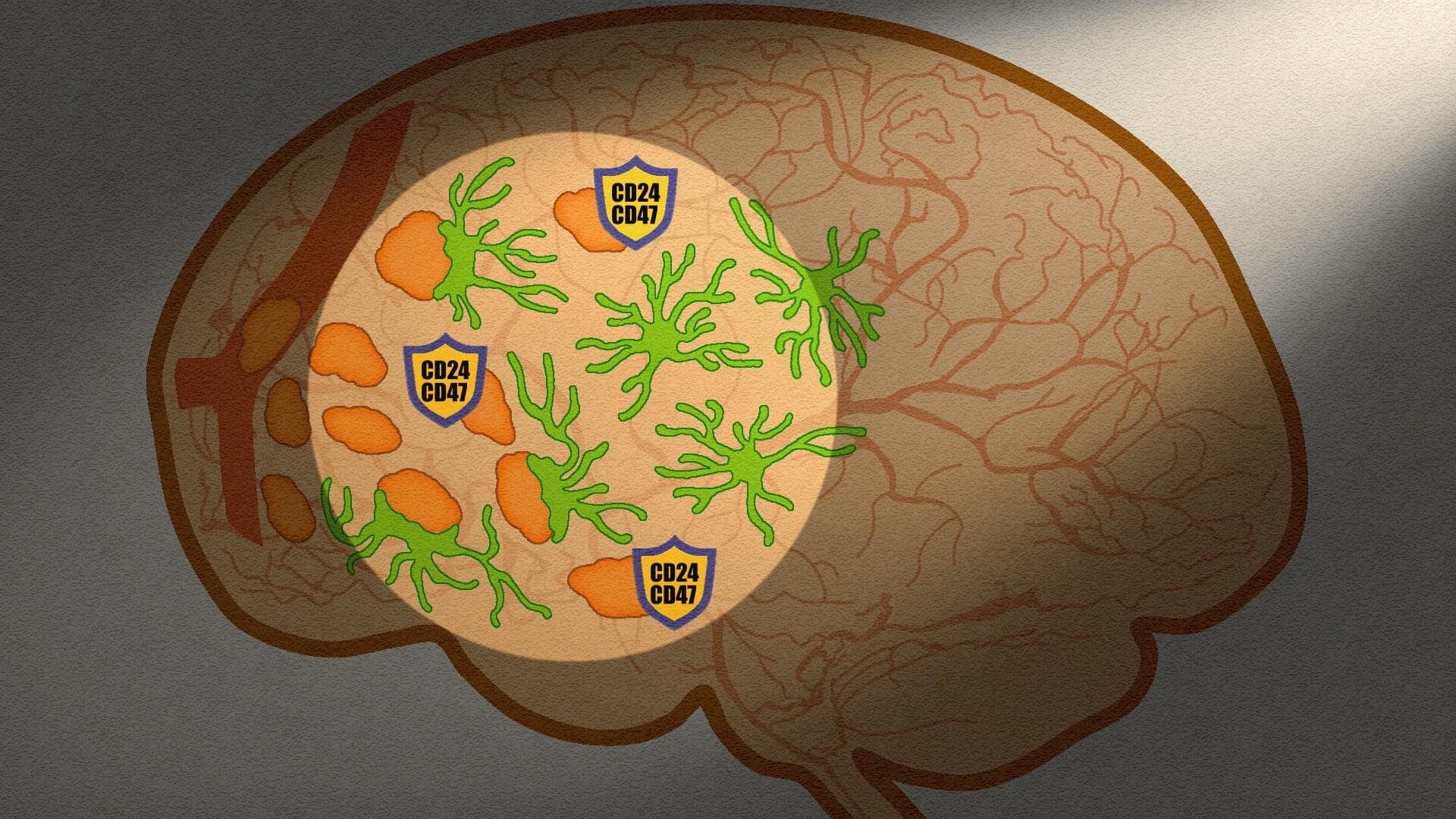
Metastasis occurs when cancer cells break away from the original tumor and travel through the bloodstream to form new tumors in other parts of the body. It is the leading cause of cancer-related death. Brain metastasis is particularly severe and affects 10–30% of patients with advanced lung, breast, and melanoma cancers.
While therapies exist for established brain tumors, there are limited strategies that directly target the very first cancer “seed cells” that enter and lodge in the brain.
Our brains, however, are equipped with immune cells called microglia that rapidly respond to pathogens and cancer cells by engulfing and digesting them. Yet scientists could not explain why microglia sometimes fail to destroy incoming seed cells because they could not watch this critical interaction in real-time in the living brain.


St. Hedwig Hospital and Charité–Universitätsmedizin Berlin researchers report that repeated mornings spent under dim indoor light in healthy young adults raised afternoon and evening cortisol and reshaped sleep in ways known from depressive illnesses.
Depressive disorders are often linked with hyperactivity of the hypothalamic–pituitary–adrenal axis, with cortisol levels that stay elevated into the afternoon and early evening instead of reaching their lowest levels, typical of early evening.
Sleep in depressive illness often carries its own fingerprint. Changes in REM sleep and a shift of slow wave sleep from the beginning of the night toward later phases have been described as biological markers of depression.
Blinking is a human reflex most often performed without thinking, like breathing. Although research on blinking is usually related to vision, a new Concordia study examines how blinking is connected to cognitive function, such as filtering out background noise to focus on what someone is trying to say to us in a crowded room.
In an article published in the journal Trends in Hearing, the researchers describe two experiments designed to measure how eye blinking changes in response to stimuli under different conditions.
They found that people naturally blink less when they are working harder to understand speech in noisy environments, suggesting that the act of blinking reflects the mental effort behind everyday listening. The research further showed that blink patterns remained stable across different lighting conditions—meaning people blinked just as much whether lighting was bright, dim or dark.
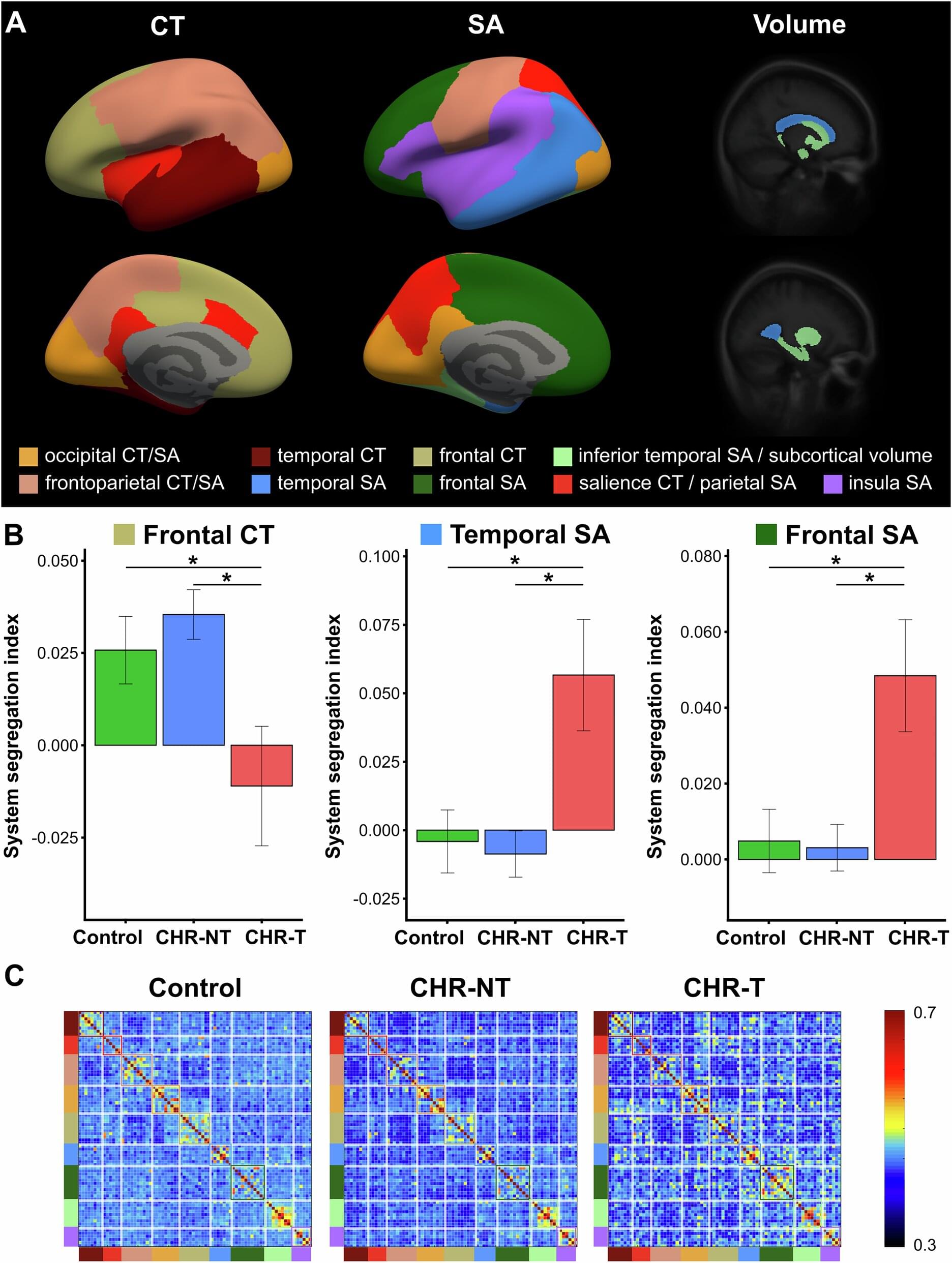
Researchers from the Yong Loo Lin School of Medicine, National University of Singapore (NUS Medicine), and NHG Health’s Institute of Mental Health (IMH) have mapped how brain networks differ in individuals at Clinical High Risk (CHR) for psychosis, providing a new perspective on the mechanisms underlying the disease onset.
Published in Molecular Psychiatry, the study utilized advanced neuroimaging methods to identify early, network-level changes in more than 3,000 individuals at varying levels of risk.
The study—led by Dr. Siwei Liu, Senior Research Scientist, and Associate Professor Juan Helen Zhou, Director, both at the Center for Translational Magnetic Resonance Research (TMR), NUS Medicine, and in collaboration with Associate Professor Jimmy Lee, Senior Consultant Psychiatrist and Clinician-Scientist at IMH—sought to determine how brain networks can reveal signs in young individuals with heightened clinical risk of developing psychosis.