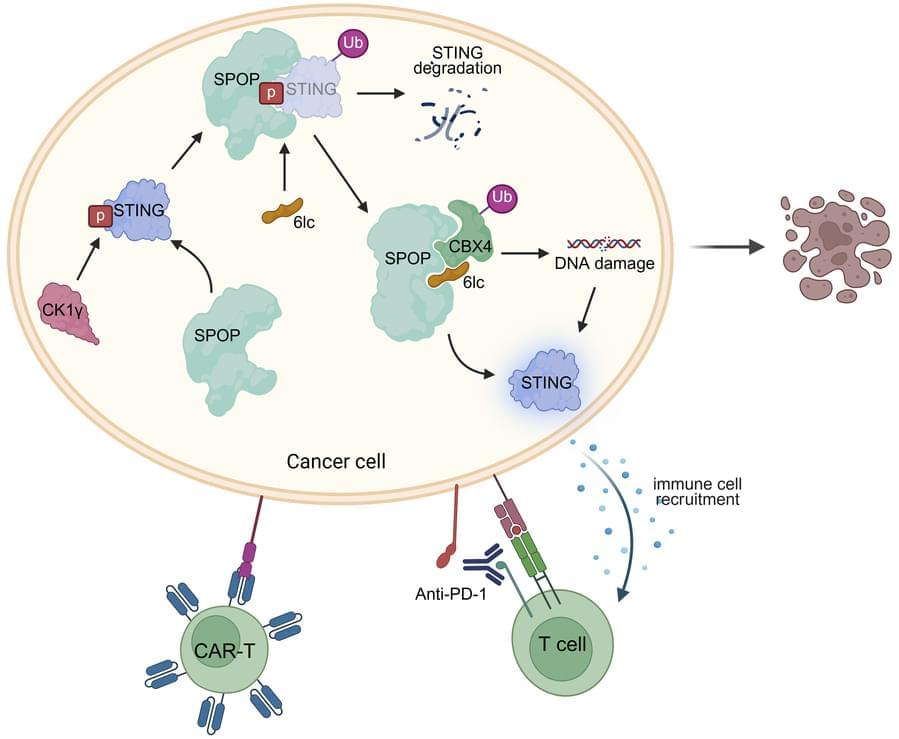
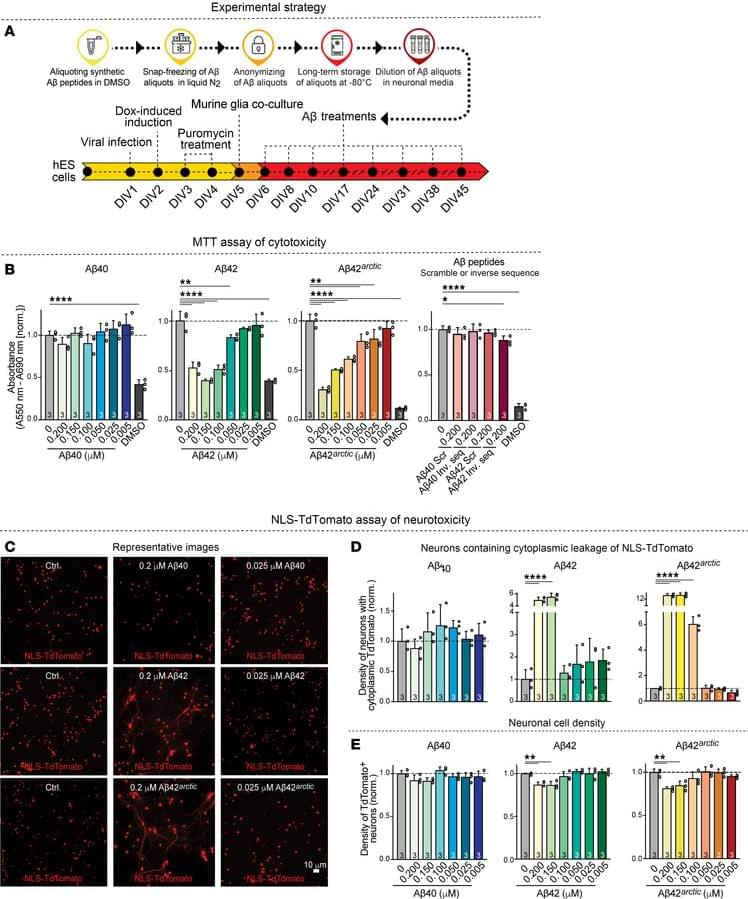
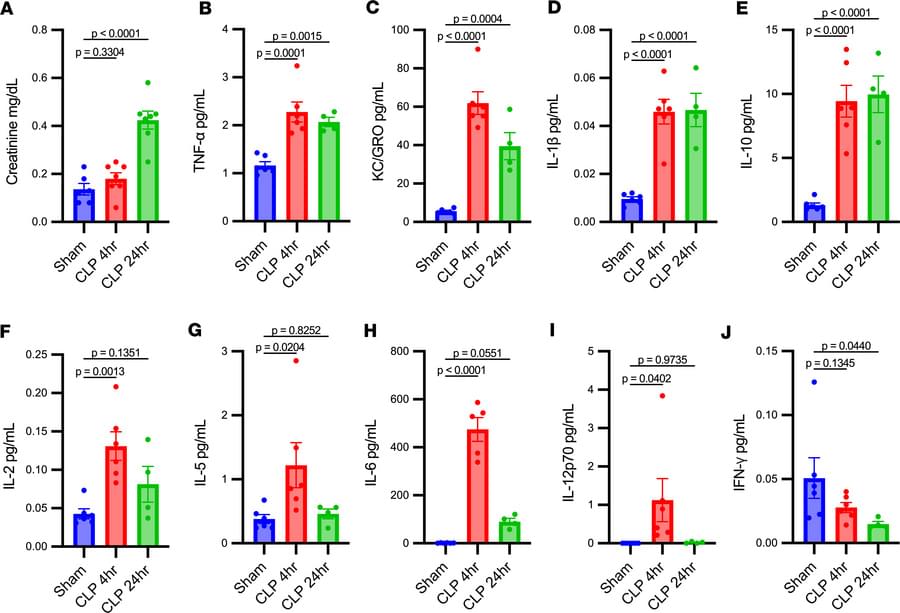
Here, Pengda Liu & team show SPOP inhibitors act as molecular glue degraders, stabilizing and activating STING to enhance immunotherapy in melanoma mouse models:
The figure shows the SPOP inhibitor 6lc reduces CBX4 and BMI1 foci, while ectopic CBX4 restores BMI1 foci and H2AX interactions.
4Department of Pharmacology.
5Division of Oncology, Department of Medicine, and.
6UNC Metabolomics and Proteomics Core, Department of Pharmacology, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.