While carbon nanotubes are the materials that have received most of the attention so far, they have proved very difficult to manufacture and control, so scientists are eager to find other compounds that could be used to create nanowires and nanotubes with equally interesting properties, but easier to handle.
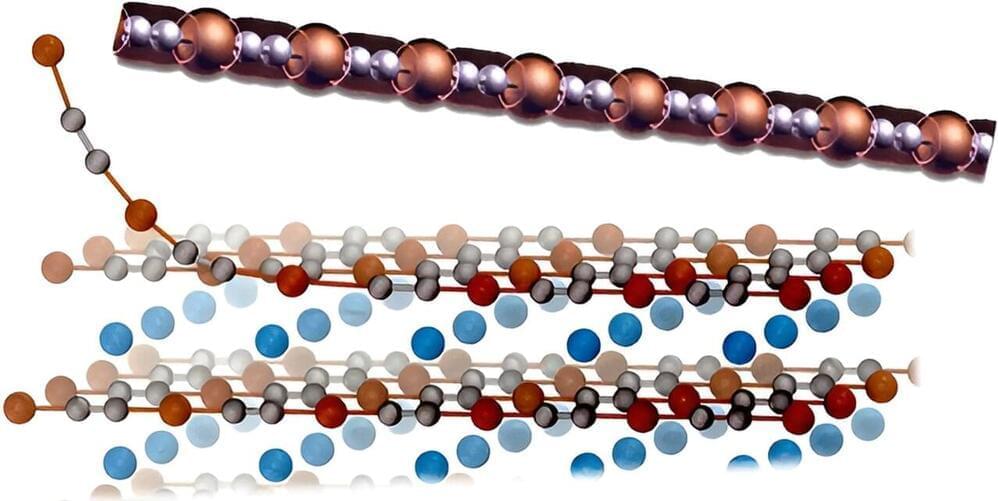
So, Chiara Cignarella, Davide Campi and Nicola Marzari thought to use computer simulations to parse known three-dimensional crystals, looking for those that—based on their structural and electronic properties —look like they could be easily “exfoliated,” essentially peeling away from them a stable 1-D structure. The same method has been successfully used in the past to study 2D materials, but this is the first application to their 1-D counterparts.
The researchers started from a collection of over 780,000 crystals, taken from various databases found in the literature and held together by van der Waals forces, the sort of weak interactions that happen when atoms are close enough for their electrons to overlap. Then they applied an algorithm that considered the spatial organization of their atoms looking for the ones that incorporated wire-like structures, and calculated how much energy would be necessary to separate that 1-D structure from the rest of the crystal.









Comments are closed.