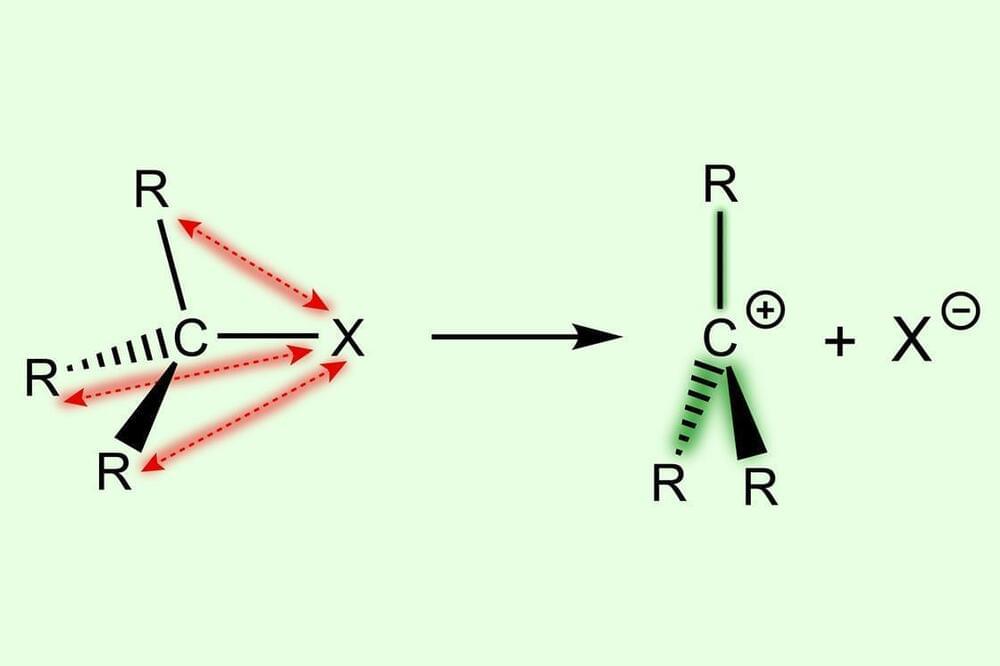
It is easier to form more substituted carbocations because of destabilisation in the parent substrate, rather than stabilisation in the reactive intermediate, new research shows.1
Many organic transformations involve carbocations as reactive intermediates. These are usually formed via a heterolytic C–X bond dissociation to give a carbocation C+ and an anion X-. Current understanding is that the bond dissociation energy decreases with increased methyl substitution because of the stabilising effect of the methyl groups, as well as relief due to steric repulsion: going from substrate to carbocation gives the substituents proportionally more room in a more substituted system. However, a team in the Netherlands, led by Matthias Bickelhaupt at VU Amsterdam, has investigated this from a different angle.








Comments are closed.