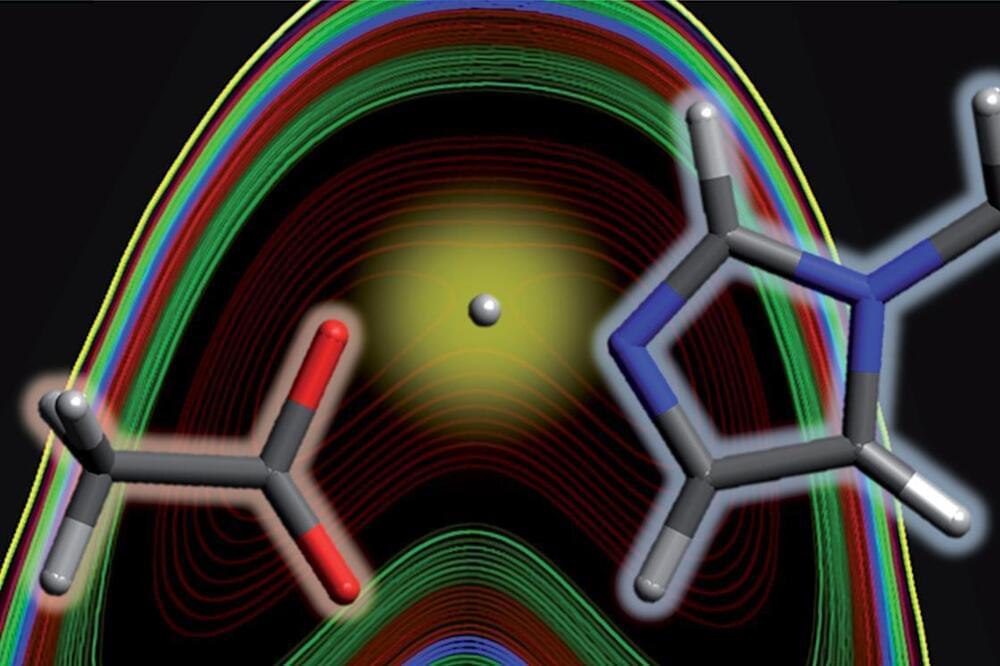
Researchers in the US have demonstrated the presence of quantum mechanical effects in acid–base interactions, challenging the Brønsted–Lowry theory. The resultant short hydrogen bond is stabilised by a delocalised proton, which rapidly shuttles between the acid and base molecules and is characterised by highly unusual spectral features.
The Brønsted–Lowry theory was proposed in 1923 and explains acid–base interactions in terms of proton transfer. This theory is one of the cornerstones of chemical understanding and is amongst the first principles taught to school students. But despite a growing appreciation for the limitations of traditional thinking, the surprising discovery of a quantum component to such fundamental reactivity was entirely serendipitous.
‘It was luck,’ admits Daniel Kuroda of Louisiana State University, one of the principal researchers involved in the study. ‘We were looking at the structure of liquids … and saw this paper [about an acid–base mixture] with close to the conductivity of sulfuric acid but no ionisation. We wanted to see what the structure was … so we started looking into the project and then realised that clearly we have something very different.’








Comments are closed.