Experiments show that effect doesn’t always follow cause in the weird world of subatomic particles, offering fresh clues about the quantum origins of space-time.


Experiments show that effect doesn’t always follow cause in the weird world of subatomic particles, offering fresh clues about the quantum origins of space-time.
Check out the Space Time Merch Store https://www.pbsspacetime.com/shopSign Up on Patreon to get access to the Space Time Discord!https://www.patreon.com/pbssp…
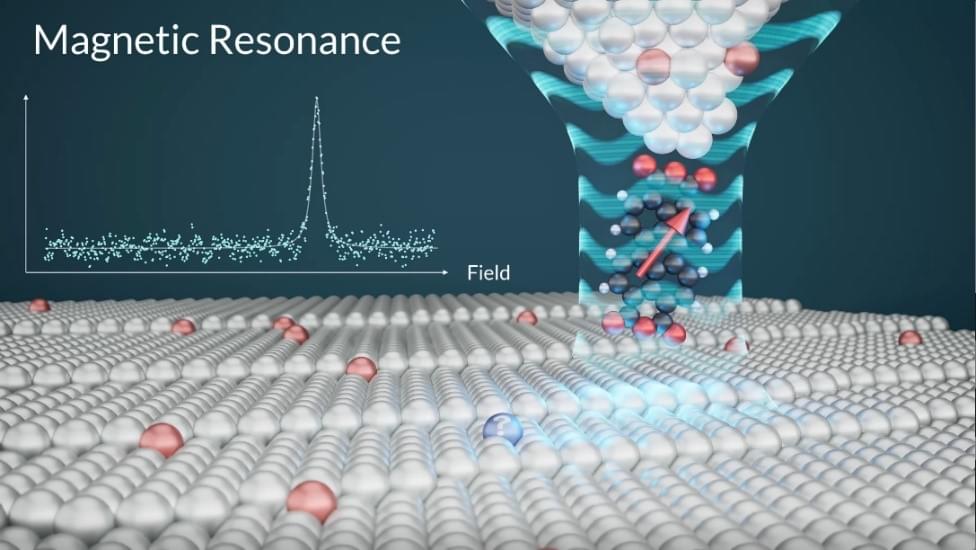
In a scientific breakthrough, an international research team from Korea’s IBS Center for Quantum Nanoscience (QNS) and Germany’s Forschungszentrum Jülich developed a quantum sensor capable of detecting minute magnetic fields at the atomic length scale. This pioneering work realizes a long-held dream of scientists: an MRI-like tool for quantum materials.
The research team utilized the expertise of bottom-up single-molecule fabrication from the Jülich group while conducting experiments at QNS, utilizing the Korean team’s leading-edge instrumentation and methodological know-how to develop the world’s first quantum sensor for the atomic world.
The diameter of an atom is a million times smaller than the thickest human hair. This makes it extremely challenging to visualize and precisely measure physical quantities like electric and magnetic fields emerging from atoms. To sense such weak fields from a single atom, the observing tool must be highly sensitive and as small as the atoms themselves.
In this thought-provoking exploration, we delve into the profound reflections of Edward Witten, a leading figure in theoretical physics. Join us as we navigate the complexities of dualities, the enigmatic nature of M-theory, and the intriguing concept of emergent space-time. Witten, the only physicist to win the prestigious Fields Medal, offers deep insights into the mathematical and physical mysteries that shape our understanding of reality. From the holographic principle to the elusive (2,0) theory, we uncover how these advanced theories interconnect and challenge our conventional perceptions. This journey is not just a deep dive into high-level physics but a philosophical quest to grasp the nature of existence itself. Read the full interview here: https://www.quantamagazine.org/edward…
#EdwardWitten #TheoreticalPhysics #StringTheory #QuantumFieldTheory #MTheory.
Become a member of this channel to enjoy benefits:
/ @artificiallyaware
Researchers at QuTech developed somersaulting spin qubits for universal quantum logic. This achievement may enable efficient control of large semiconductor qubit arrays. The research group published their demonstration of hopping spins in Nature Communications and their work on somersaulting spins in Science.
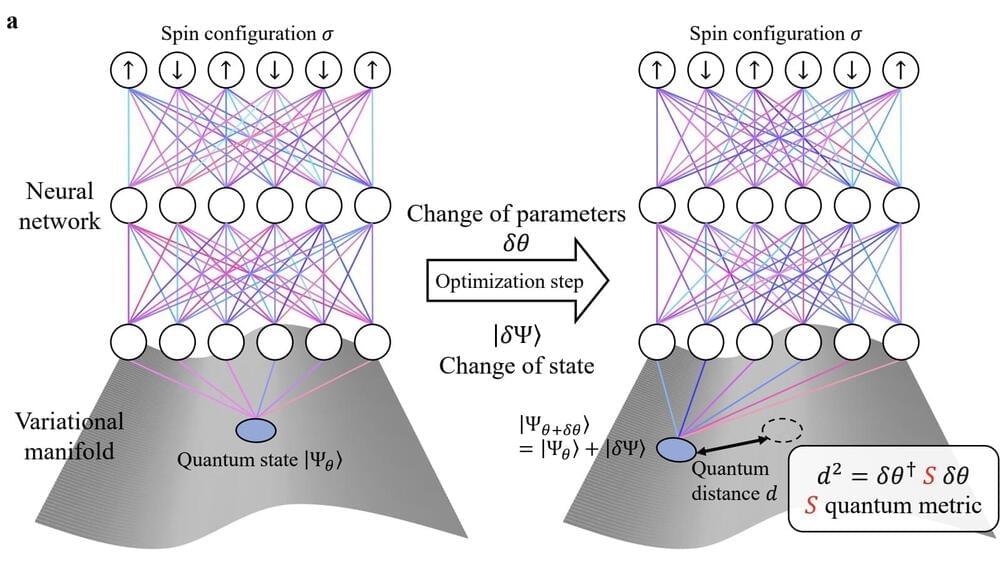
Over the past decades, computer scientists have developed various computing tools that could help to solve challenges in quantum physics. These include large-scale deep neural networks that can be trained to predict the ground states of quantum systems. This method is now referred to as neural quantum states (NQSs).
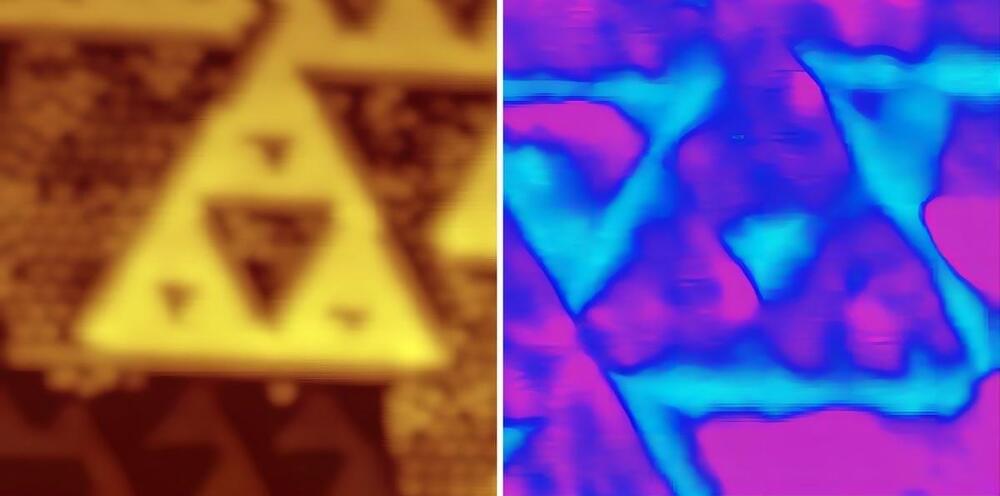
Topological insulators, capable of transmitting electricity without loss, may function in fractional dimensions such as 1.58. This breakthrough, combined with room-temperature operability, paves the way for advancements in quantum computing and energy efficiency through fractal structures.
What if we could find a way to make electric currents flow, without energy loss? A promising approach for this involves using materials known as topological insulators. They are known to exist in one (wire), two (sheet) and three (cube) dimensions; all with different possible applications in electronic devices. Theoretical physicists at Utrecht University, together with experimentalists at Shanghai Jiao Tong University, have discovered that topological insulators may also exist at 1.58 dimensions, and that these could be used for energy-efficient information processing. Their study was published recently in Nature Physics.
Classical bits, the units of computer operation, are based on electric currents: electrons running means 1, no electrons running means 0. With a combination of 0s and 1s, one can build all the devices that you use in your daily life, from cellphones to computers. However, while running, these electrons meet defects and impurities in the material, and lose energy. This is what happens when your device gets warm: the energy is converted into heat, and so your battery is drained faster.

A novel quantum sensor with exceptional resolution transforms atomic-level material analysis, paving the way for advancements in quantum technologies and sciences.
In a scientific breakthrough, an international research team from Germany’s Forschungszentrum Jülich and Korea’s IBS Center for Quantum Nanoscience (QNS) developed a quantum sensor capable of detecting minute magnetic fields at the atomic length scale. This pioneering work realizes a long-held dream of scientists: an MRI-like tool for quantum materials.
Quantum Sensor Development

Researchers at California State Polytechnic University (CalPoly), Pomona are carrying out a series of quantum physics experiments expected to provide strong scientific evidence that we live in a computer simulated virtual reality.
Devised by former NASA physicist Thomas Campbell, the five experiments are variations of the double-slit and delayed-choice quantum eraser experiments, which explore the conditions under which quantum objects ‘collapse’ from a probabilistic wavefunction to a defined particle. In line with the Copenhagen Interpretation of quantum mechanics, Campbell attributes a fundamental role to measurement, but extends it to human observers. In his view, quantum mechanics shows that the physical world is a virtual reality simulation that is computed for our consciousness on demand. In essence, what you do not see does not exist.
Campbell’s quantum experiments have been designed to reveal the interactive mechanism by which nature probabilistically generates our experience of the physical world. Herein, Campbell asserts that, like a videogame, the universe is generated as needed for the player and does not exist independent of observation.
While multiple quantum experiments have pointed to the probabilistic and informational nature of reality, Campbell’s experiments are the first to investigate the connection between consciousness and simulation theory. These experiments are based on Campbell’s paper ‘On Testing the Simulation Theory’ originally published in the International Journal of Quantum Foundations in 2017.
Paradigm-shifting consequences
Importantly, Campbell’s version of the simulation hypothesis differs from the ‘ancestor simulation’ thought experiment popularized by philosopher Dr. Nick Bostrom. “Contrary to what Bostrom postulates, the idea here is that consciousness is not a product of the simulation — it is fundamental to reality,” Campbell explains. “If all five experiments work as expected, this will challenge the conventional understanding of reality and uncover profound connections between consciousness and the cosmos.” The first experiment is currently being carried out by two independent teams of researchers — One at California State Polytechnic University (Pomona) headed by Dr. Farbod Khoshnoud, and the other at a top-tier Canadian university that has chosen to participate anonymously at this time.
To learn more, or to follow their progress visit Testing the Hypothesis, a platform bringing together all relevant information about Campbell’s experiments, including a detailed explanation of each.