When it comes to memories, the nose knows. Find out why smell can trigger memories, both good and bad.



Stanford Medicine researchers found cells that keep a speech-linked protein called FOXP2 from clumping; its tricks could break apart clumps of proteins that cause devastating brain diseases.
This week, researchers reported the discovery of four Late Bronze Age stone megastructures likely used for trapping herds of wild animals. Physicists have proven that a central law of thermodynamics does not apply to atomic-scale objects that are linked via quantum correlation. And two Australian Ph.D. students coded a software solution for the James Webb Space Telescope’s Aperture Masking Interferometer, which has been producing blurry images.
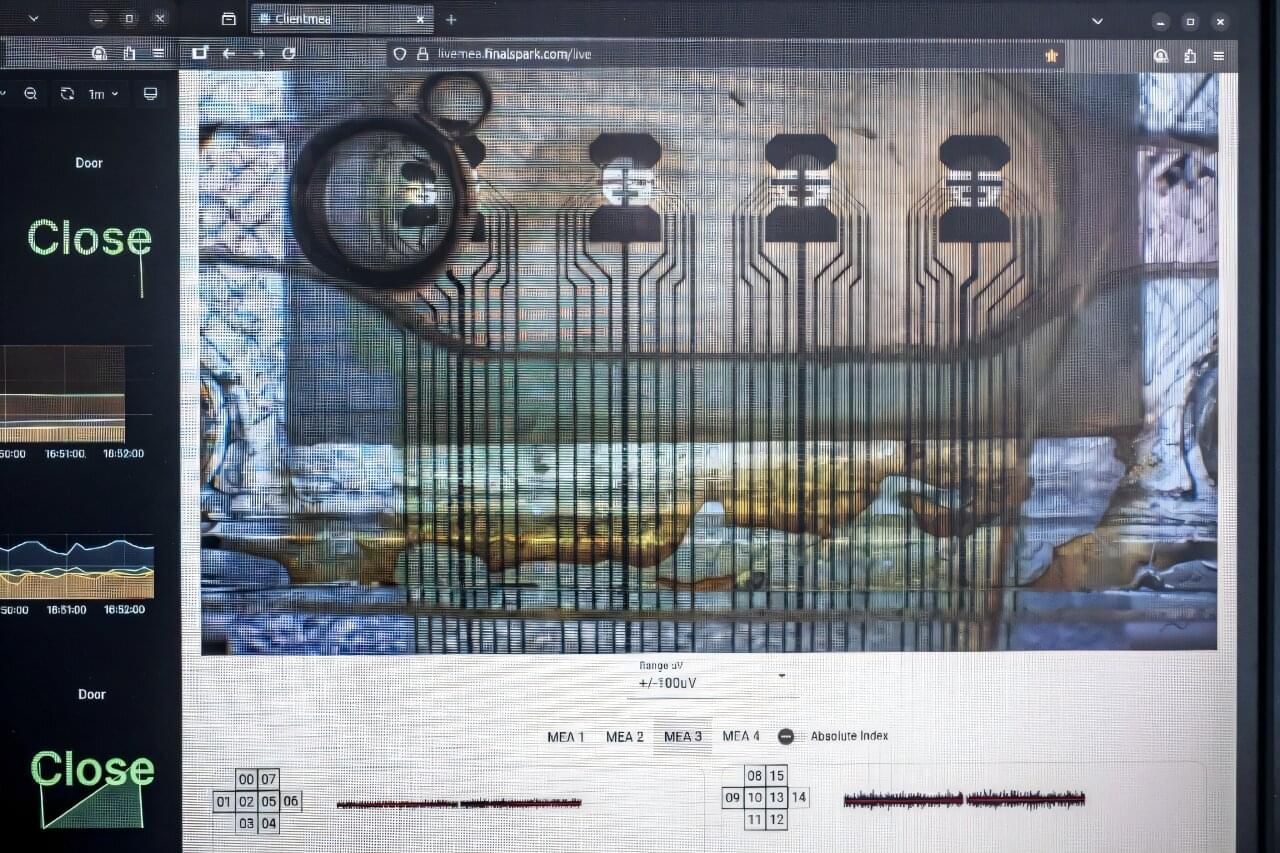
Additionally, researchers are networking tiny human brain organoids into a computing substrate; evolutionary biologists have proposed that environmental lead exposure may have influenced early human brain evolution; and physicists have provided a predictive model to explain accelerating universal expansion without dark matter:

The number of people living with dementia worldwide was estimated at 57 million in 2021 with nearly 10 million new cases recorded each year. In the U.S., dementia impacts more than 6 million lives, and the number of new cases is expected to double over the next few decades, according to a 2025 study. Despite advancements in the field, a full understanding of disease-causing mechanisms is still lacking.
To address this gap, Rice University researchers and collaborators at Boston University have developed a computational tool that can help identify which specific types of cells in the body are genetically linked to complex human traits and diseases, including in forms of dementia such as Alzheimer’s and Parkinson’s.
Known as “Single-cell Expression Integration System for Mapping genetically implicated Cell types,” or seismic, the tool helped the team hone in on genetic vulnerabilities in memory-making brain cells that link them to Alzheimer’s ⎯ the first to establish an association based on a genetic link between the disease and these specific neurons. The algorithm outperforms existing tools for identifying cell types that are potentially relevant in complex diseases and is applicable in disease contexts beyond dementia.

An international consortium of researchers has created the largest-ever database compiling records of brain activity during sleep and dream reports. One of the first analyses of the database confirmed that dreams do not occur only during REM sleep, but also during deeper and calmer NREM stages. In these cases, brain activity resembles wakefulness more than deep sleep, as if the brain were “partially awake.”
One third of a healthy adult’s life is spent sleeping, and a significant portion of that time is spent dreaming. Throughout the night, during any sleep stage, subjective conscious experiences, what we call dreams, can repeatedly occur.
Interest in dreams dates back thousands of years, from ancient Egypt to ancient Mesopotamia and ancient Greece, and spans many cultures and traditions.
Researchers have discovered how a surface protein on brain cells, called Aplp1, can play a role in spreading material responsible for Parkinson’s disease from cell to cell in the brain.
Promisingly, an FDA-approved cancer drug that targets another protein – Lag3 – which interacts with Aplp1 – was found to block this process in mice. This suggests a potential treatment for Parkinson’s may already exist.
In a paper published last year, an international team of scientists detailed how the two proteins work together to help toxic clumps of alpha-synuclein protein get into brain cells.
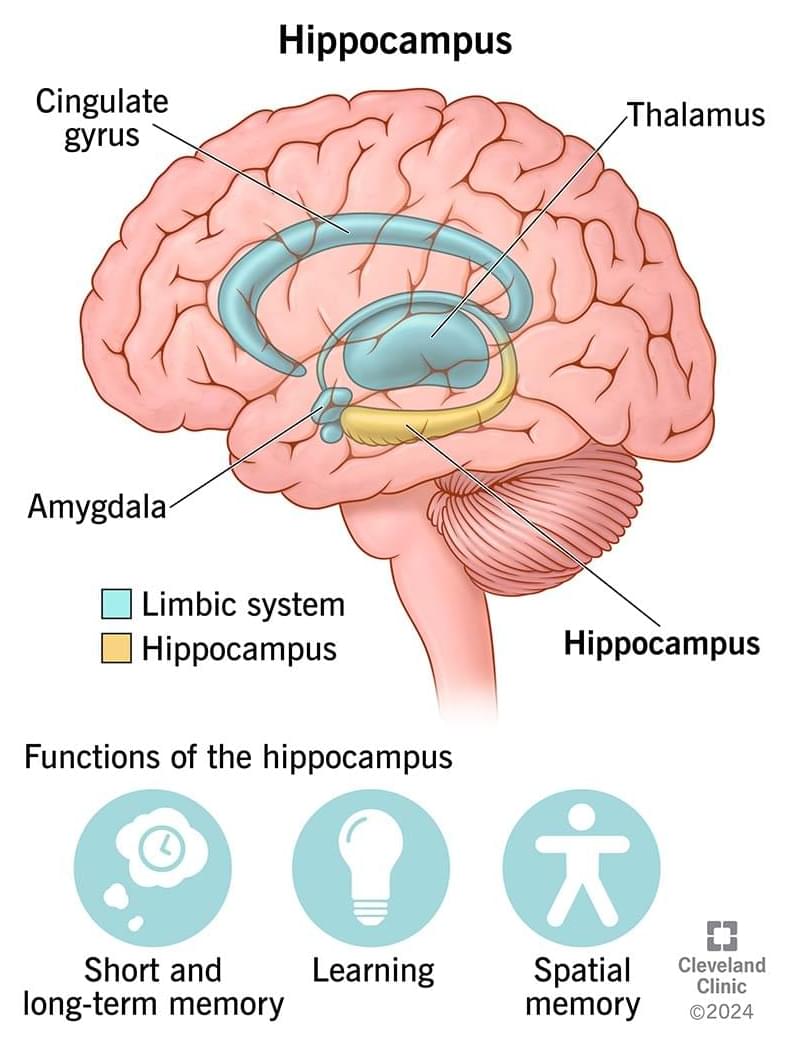
It converts short-term memories into long-term memories by organizing, storing and retrieving memories within your brain. Your also helps you learn more about your environment (spatial memory), so you’re aware of what’s around you, as well as remembering what words to say (verbal memory).
You have a on the left and right side of your brain, located within the temporal lobe.
The is part of your limbic system. This is a group of brain structures that regulate your smells, emotions, memories and autonomic behaviors (such as heart rate, breathing, sweating, etc.).


A large international study involving nearly 700 participants reveals that women with a precursor condition to Parkinson’s disease show significantly less brain atrophy—decreased cortical thickness in the brain—than men, despite similar clinical severity. This discovery, published in the journal Nature Communications, could lead scientists to explore the role that hormones might play in treating the disease.
Isolated REM sleep behavior disorder is characterized by violent movements during sleep, where people literally “act out” their dreams. Far from being harmless, this disorder is the most reliable early warning sign of neurodegenerative diseases caused by the accumulation of a toxic protein in the brain: more than 70% of affected individuals will eventually develop Parkinson’s disease, Lewy body dementia, or, more rarely, multiple system atrophy (a disease affecting multiple body systems).
“This sleep disorder offers a unique window of opportunity to study the mechanisms of neurodegeneration before major motor or cognitive symptoms appear,” explains Shady Rahayel, professor at UdeM’s Faculty of Medicine and leader of this study.