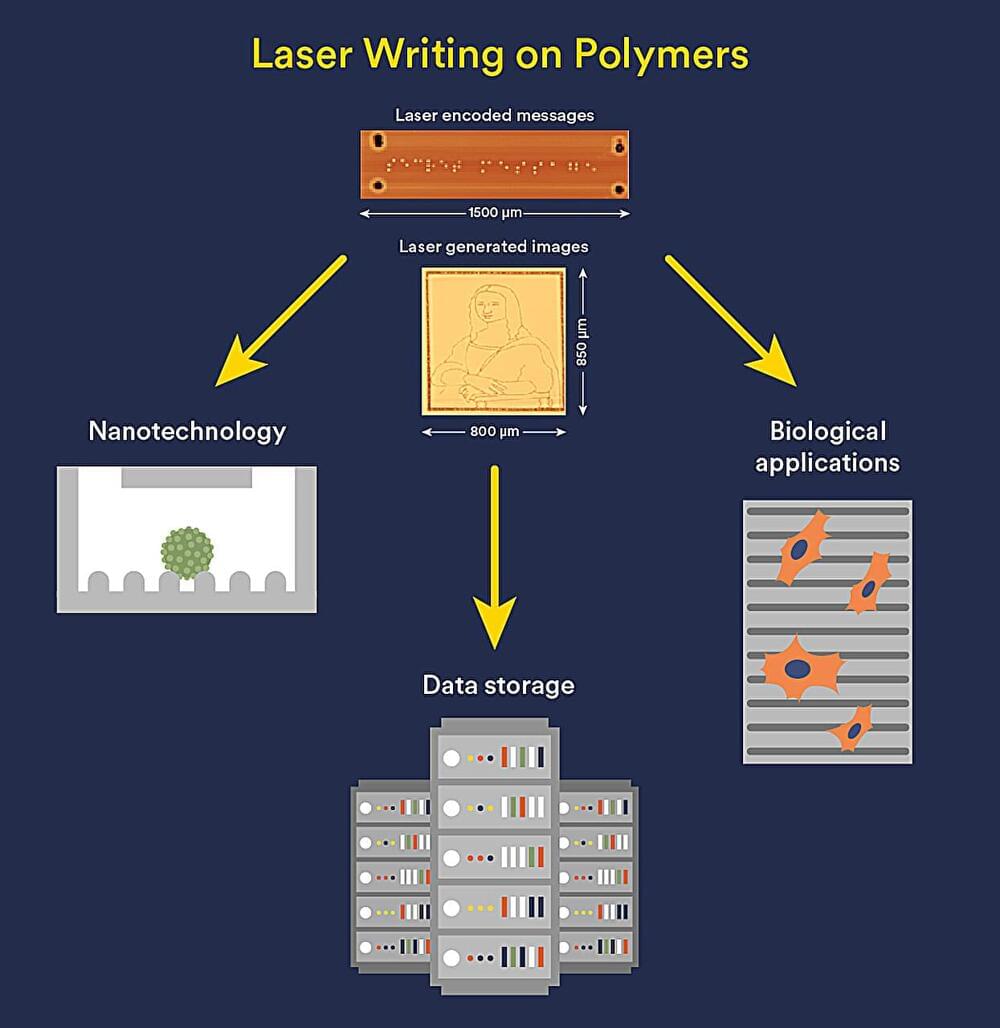
Now Flinders University researchers have discovered a light-responsive, inexpensive sulfur-derived polymer receptive to low power, visible light lasers—which promises a more affordable and safer production method in nanotech, chemical science and patterning surfaces in biological applications.
Details of the novel system have just been published in Angewandte Chemie International Edition, featuring a laser-etched version of the famous “Mona Lisa” painting and micro-Braille printing even smaller than a pin head.
“This could be a way to reduce the need for expensive, specialized equipment, including high-power lasers with hazardous radiation risk, while also using more sustainable materials. For instance, the key polymer is made from low-cost elemental sulfur, an industrial byproduct, and either cyclopentadiene or dicyclopentadiene,” says Matthew Flinders Professor of Chemistry Justin Chalker, from the Flinders University.