The new material could be key to finally building a successful fusion reactor.


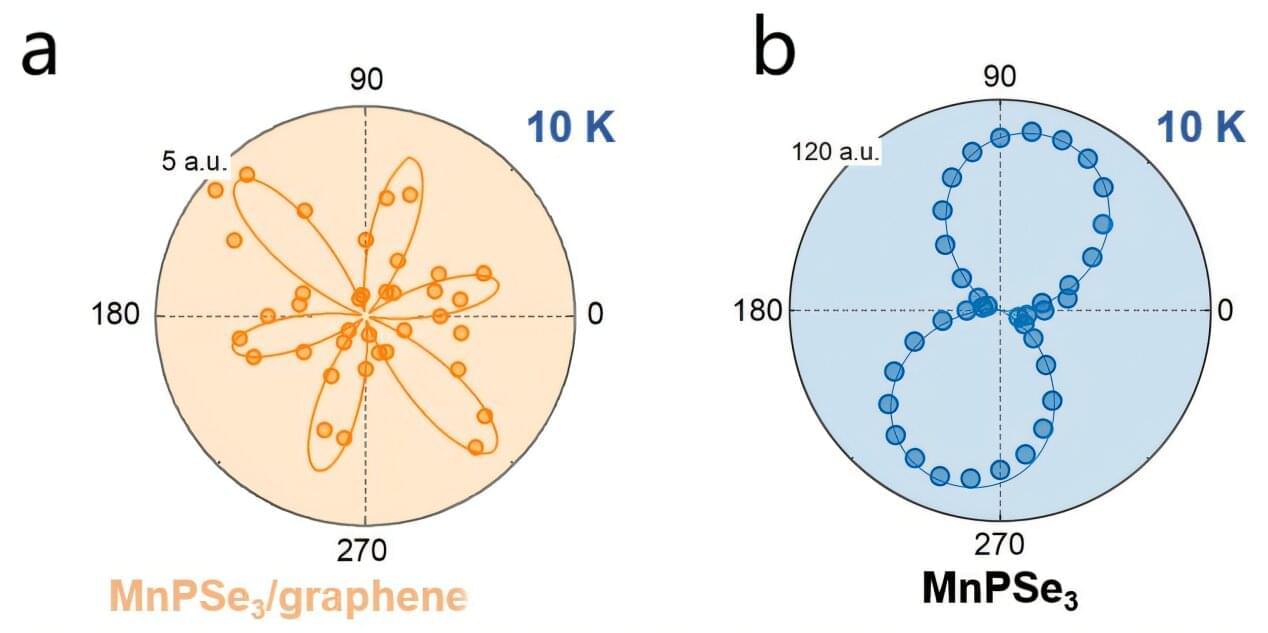
A research group recently discovered the disappearance of nonreciprocal second harmonic generation (SHG) in MnPSe₃ when integrated into a two-dimensional (2D) antiferromagnetic MnPSe₃/graphene heterojunction.
The research, published in Nano Letters, highlights the role of interfacial magnon-plasmon coupling in this phenomenon.
2D van der Waals magnetic/non-magnetic heterojunctions hold significant promise for spintronic devices. Achieving these functionalities hinges on the interfacial proximity effect, a critical factor. However, detecting the proximity effect in 2D antiferromagnetic/non-magnetic heterojunctions presents considerable challenges, due to the small size and weak signals associated with these structures.
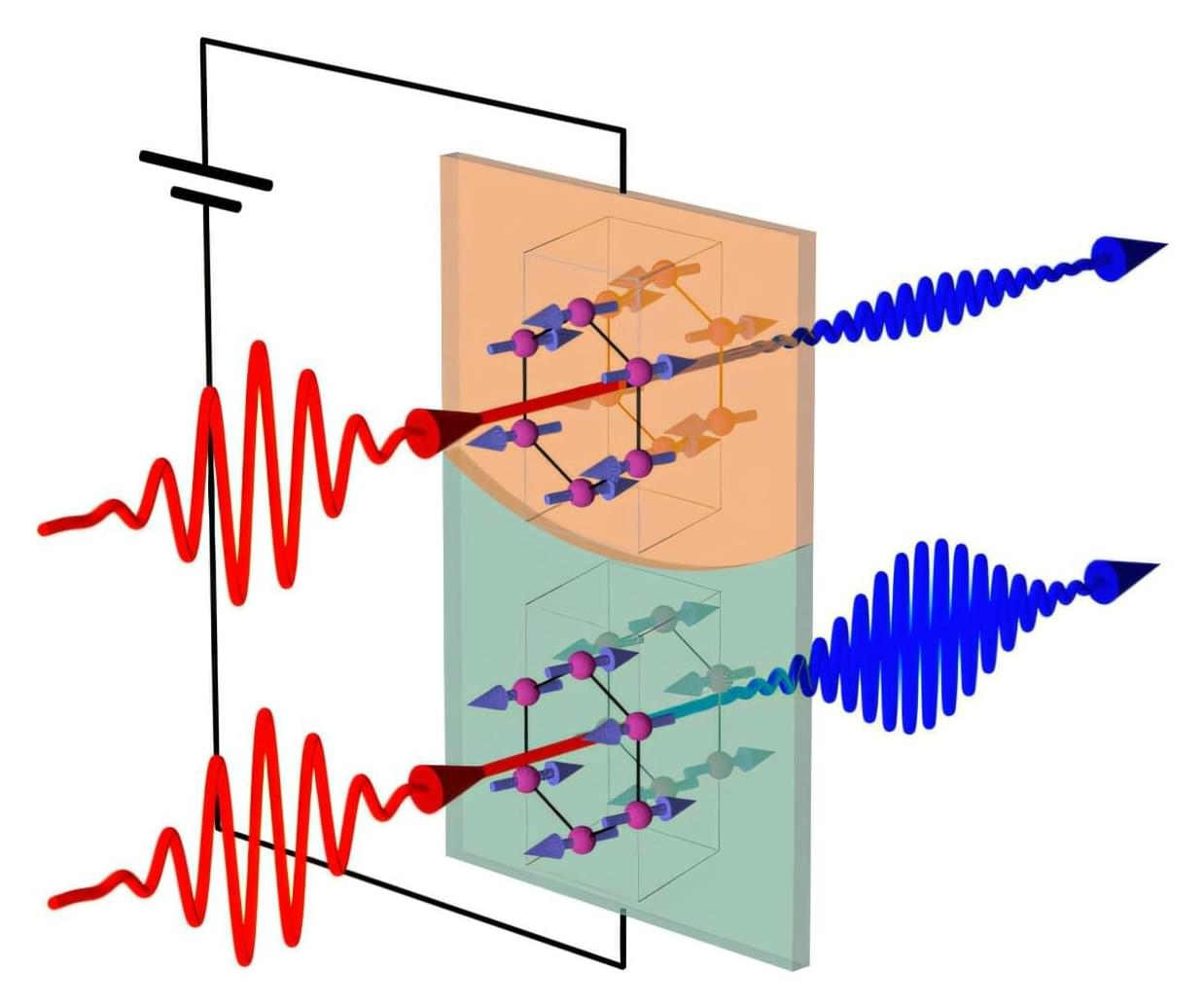
A new trick for illuminating the internal ordering within a special type of magnet could help engineers build better memory-storage devices. Developed by RIKEN physicists, this technique could make memory devices less corruptible.
The work is published in the journal Nature Communications.
Conventional hard disks are based on ferromagnets—materials in which the magnetic dipoles, or spins, associated with each atom all point in the same direction. This alignment gives the material a net magnetic field. Data is stored by creating different magnetization patterns across the material.
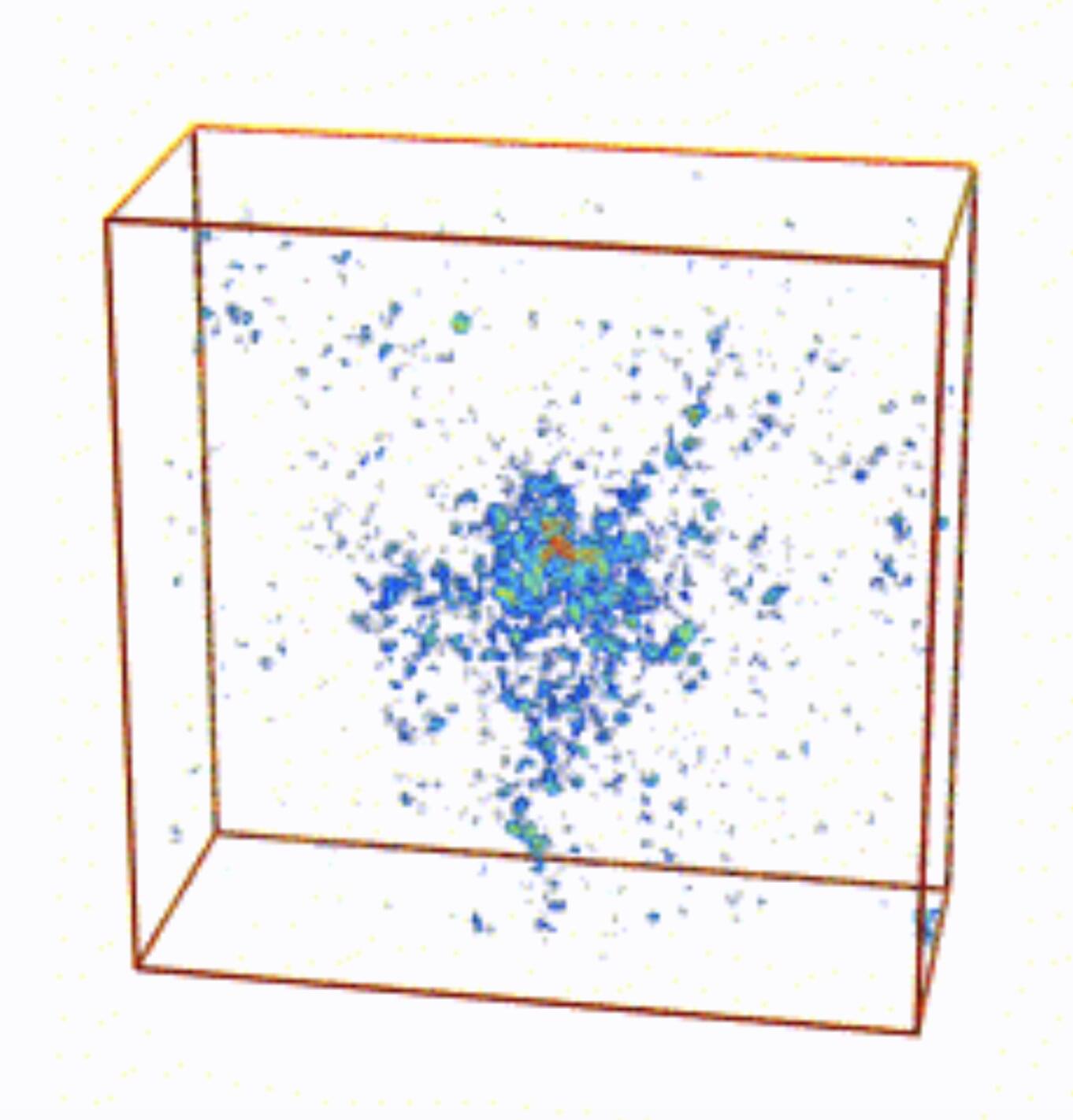
The Anderson transition is a phase transition that occurs in disordered systems, which entails a shift from a diffusive state (i.e., in which waves or particles are spread out) to a localized state, in which they are trapped in specific regions. This state was first studied by physicist Philip W. Anderson, who examined the arrangement of electrons in disordered solids, yet it was later found to also apply to the propagation of light and other waves.
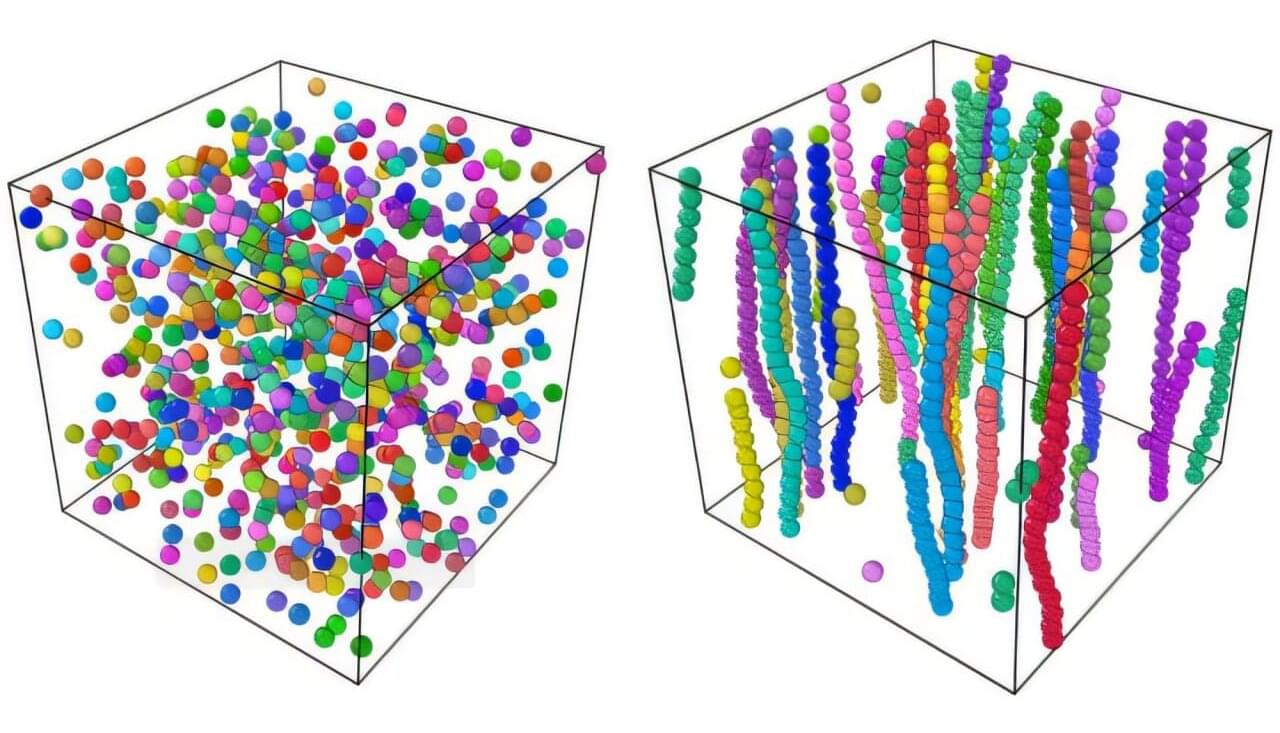
Researchers at Missouri University of Science & Technology, Yale University, and Grenoble Alpes University in France recently set out to further explore the Anderson transition for light (i.e., electromagnetic waves) in 3D disordered systems.
Their paper, published in Physical Review Letters, outlines the simulation of light wave transport in an arrangement of perfect-electric-conducting (PEC) spheres, materials that reflect electromagnetic waves.
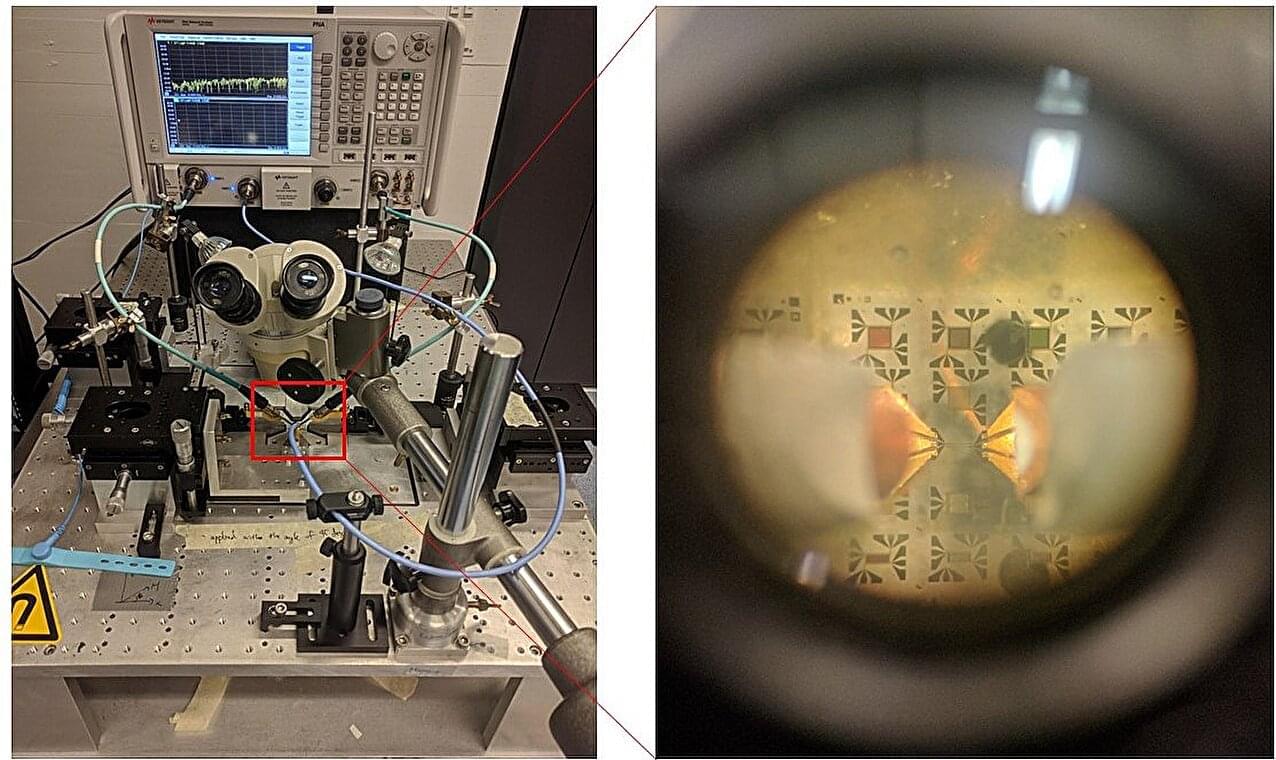
A Korean research team has succeeded in securing a basic technology for further improving the completeness level of neuromorphic devices. Their paper is published in the journal Nature Communications.
Researchers from the Korea Research Institute of Standards and Science observed the fine structure of the magnon, which is attracting attention as a key material for neuromorphic devices. As areas that are approximately 1,000 times finer than before were observed successfully, it is expected that the results will enable the design of more sophisticated neuromorphic devices.
Neuromorphic devices are next-generation semiconductors designed to mimic the structure of the human brain. They process information by mimicking the way neurons generate signals and transmit them to other neurons through synapses.
Researchers at the University of Bristol have made a breakthrough in the development of “life-like” synthetic materials which are able to move by themselves like worms.
Scientists have been investigating a new class of materials called “active matter,” which could be used for various applications from drug delivery to self-healing materials.
Compared to inanimate matter—the sort of motionless materials we come across in our lives every day, such as plastic and wood—active matter can show fascinating life-like behavior.
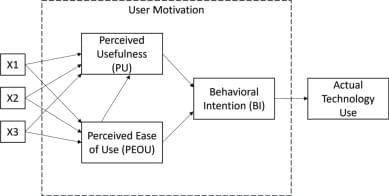
The rise of generative AI has been a major disruptive force in academia. Academics are concerned about its impact on student learning. Students can use generative AI technologies, such as ChatGPT, to complete many academic tasks on their behalf. This could lead to poor academic outcomes as students use ChatGPT to complete assessments, rather than engaging with the learning material. One particularly vulnerable academic activity is academic writing. This paper reports the results of an active learning intervention where ChatGPT was used by students to write an academic paper. The resultant papers were then analysed and critiqued by students to highlight the weaknesses of such AI-produced papers. The research used the Technology Acceptance Model to measure changing student perceptions about the usefulness and ease of use of ChatGPT in the creation of academic text.

A team of physicists at Fudan University, working with colleagues from Henan University, both in China, and from Nanyang Technological University, in Singapore and Donostia International Physics Center, in Spain, has developed a way to generate topological structures in surface water using gravity water waves. In their study published in Nature, the group used their technique to generate structures such as wave vortices, skyrmions and Möbius strips.
Prior research has shown that various types of waves can be used to achieve desired goals in a variety of applications; optical tweezers, for example, are used to capture and manipulate individual or groups of molecules to create materials or test molecular properties. Sound waves can be used to control much larger particles, or even objects, such as the membrane in a stereo speaker.
For this new study, the research team found a way to generate topological structures on the surface of water by taking advantage of the noise that develops when waves are laid on top of one another, giving them topological properties that can be used to generate wave fields.