In semiconductor device applications, there is an increasing demand for semiconductors with very high carrier concentrations. The semiconductor material parameters, namely carrier density and mobility, primarily determine device performance. Hence, it is important to accurately characterize the carrier density and mobility of a semiconductor for the development of its device applications.
The use of THz waves, or electromagnetic radiation with wavelengths of around 300 µm and frequency of about 1 THz, in the nondestructive testing of semiconductors has been continuously expanding. Free carriers in a material absorb THz radiation, which makes it possible to estimate the electrical properties of semiconductors using THz waves.
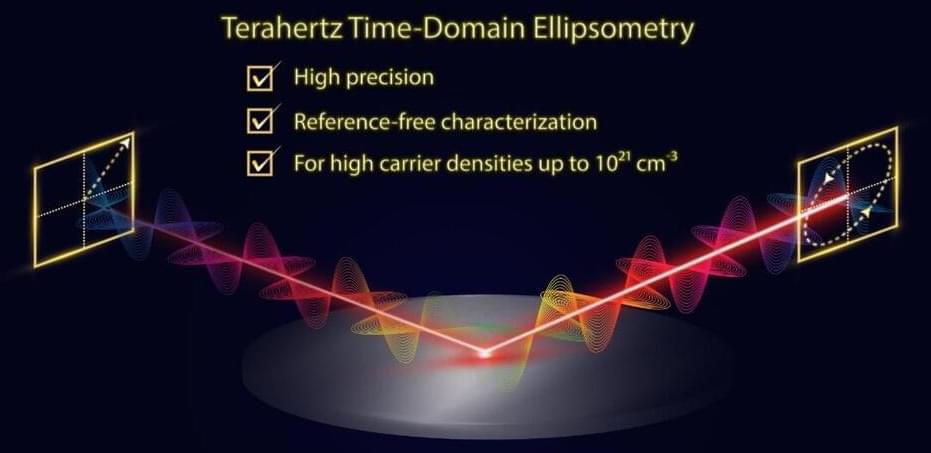
Researchers at Osaka University, in collaboration with Nippo Precision Co., Ltd., developed a THz time-domain ellipsometry system (Tera Evaluator) that extends the range of carrier concentrations measurable by THz waves up to ~1020 cm-3 and potentially higher by improving the precision of said optical technique. In THz time-domain ellipsometry, linearly polarized THz pulses are incident on a sample and the electric field strength of the reflected THz waves as a function of time is measured. Specifically, the reflected waves polarized in the direction parallel ℗ and perpendicular (s) to the plane of incidence are of interest. The ratio of the p-and s-polarization components yields information on the electric permittivity of the sample, allowing for the evaluation of the carrier density and mobility. As such, unlike THz time-domain spectroscopy, THz time-domain ellipsometry does not require reference measurements through an aperture or standard mirror.