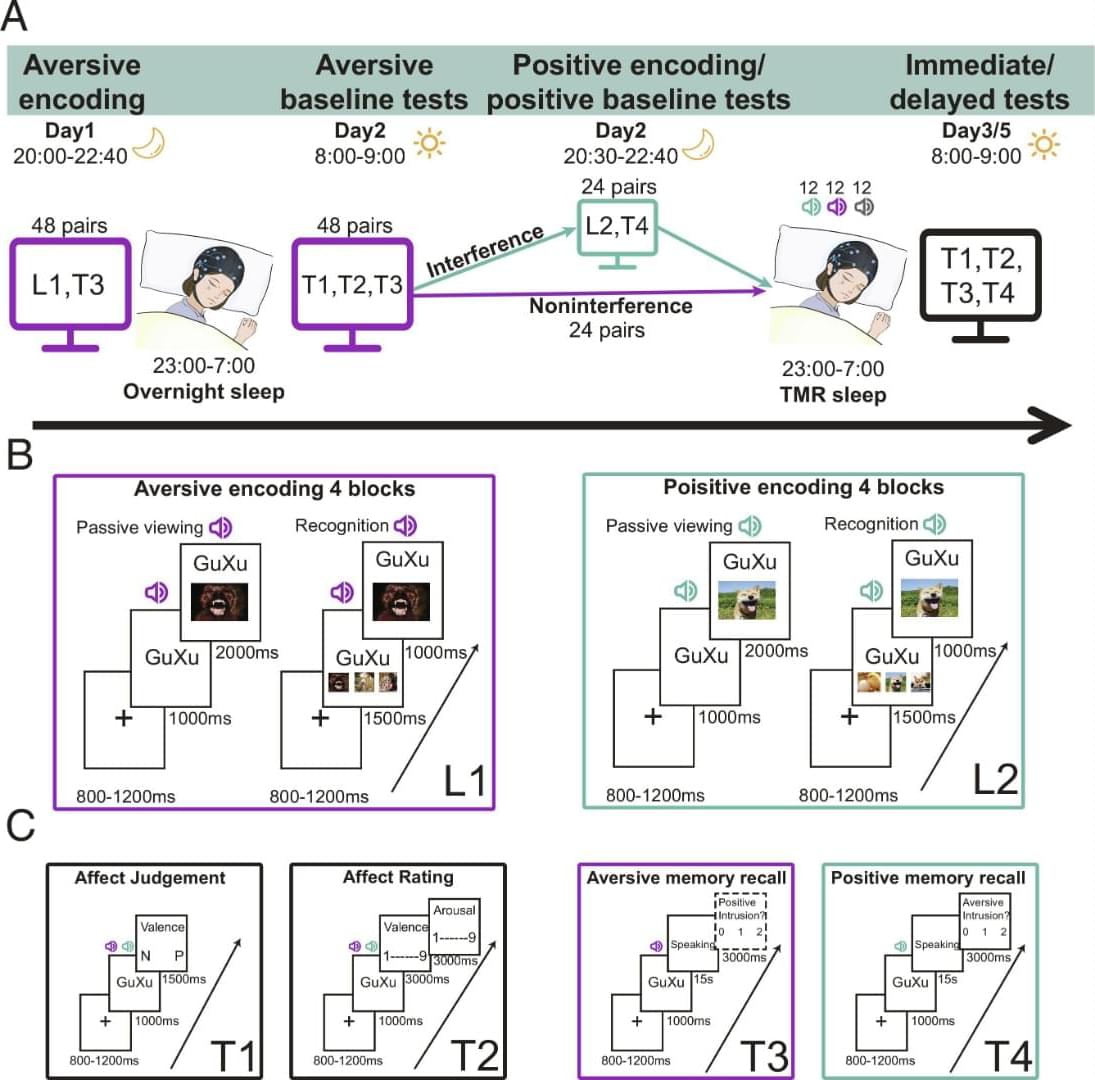
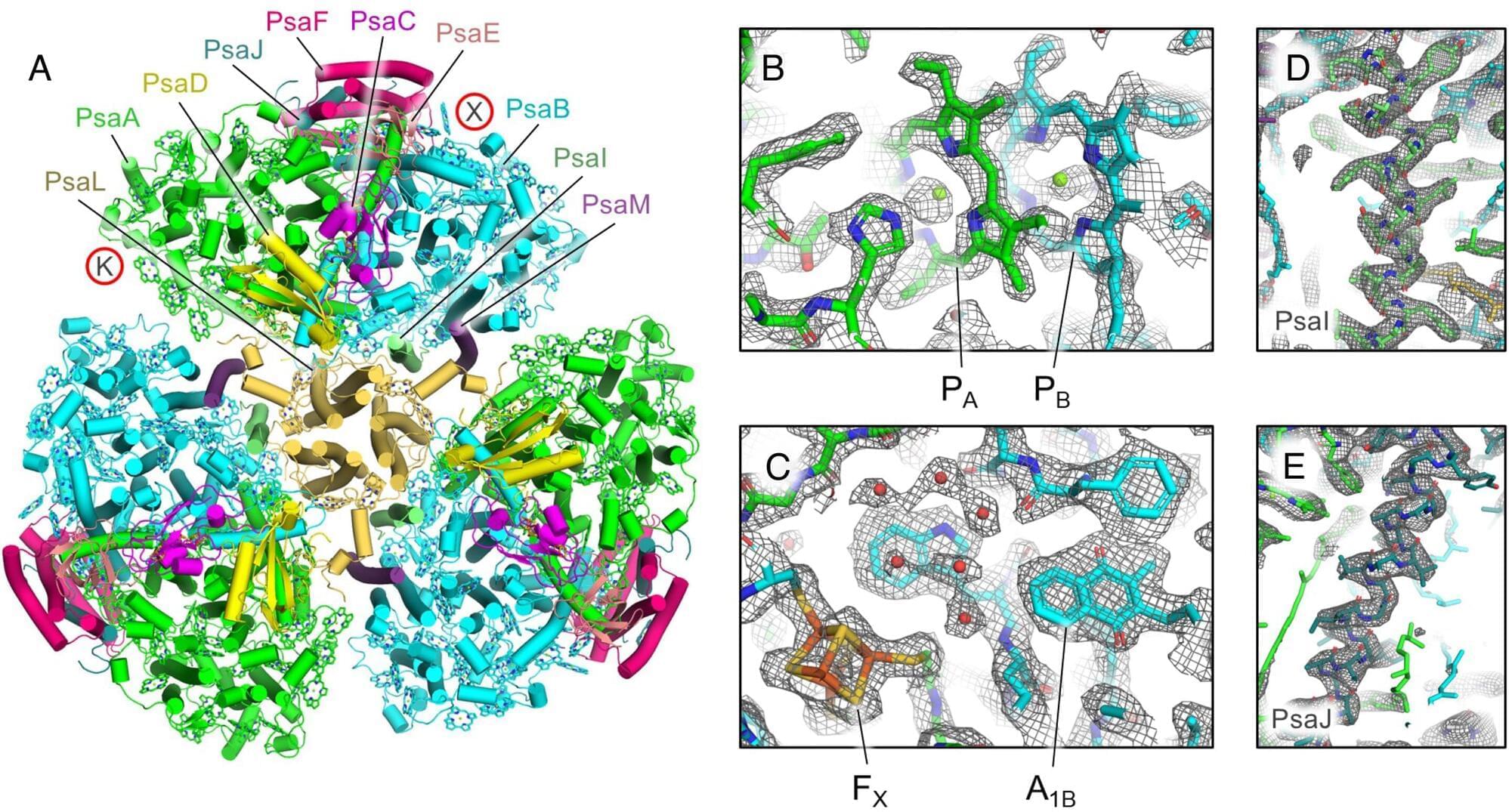
Our behavioral findings indicated that TMR weakened earlier acquired aversive memories while increasing positive memory intrusions in the interference condition. To examine how TMR reactivated aversive and positive memories during NREM sleep, we extracted cue-locked, time-frequency resolved EEG responses in the interference and noninterference conditions, and compared them with the EEG responses elicited by control sounds.
We found that when compared to the control sounds that did not involve any memory pairs before sleep, both interference and noninterference memory cues increased EEG power across the delta, theta, sigma, and beta bands in frontal and central areas (Pclusters < 0.01, corrected for multiple comparisons across time, frequency, and space, Fig. 4 A–D). However, when contrasting interference with noninterference memory cues, we did not identify any significant clusters (Pclusters 0.05). These findings suggested that delta-theta and sigma-beta power increases may indicate memory reactivation during sleep.
We next examined whether cue-elicited theta and beta power were associated with subsequent memory accuracies (i.e., remembered vs. forgotten) for individual positive or aversive stimulus in the interference condition, given the relationship between theta activity and emotional processing (19), and between beta activity and memory interference during sleep (27, 34). Employing BLMM across all channels revealed that the cue-elicited theta power over the right central-parietal region (FC5, C2, C4, CP2, CP4, TP7) was significantly higher for subsequently remembered than for forgotten positive memories (mediandiff = 1.39, 95% HDI [0.32, 2.43], Fig. 4E). For aversive memories, a few channels’ (Fp2, F6, C5) theta power was higher for remembered than for forgotten aversive memories (mediandiff = 1.04, 95% HDI [0.16, 1.86]; Fig. 4F).