Scientists discover fungus species in Chernobyl nuclear zone have mutated to feed on radiation:
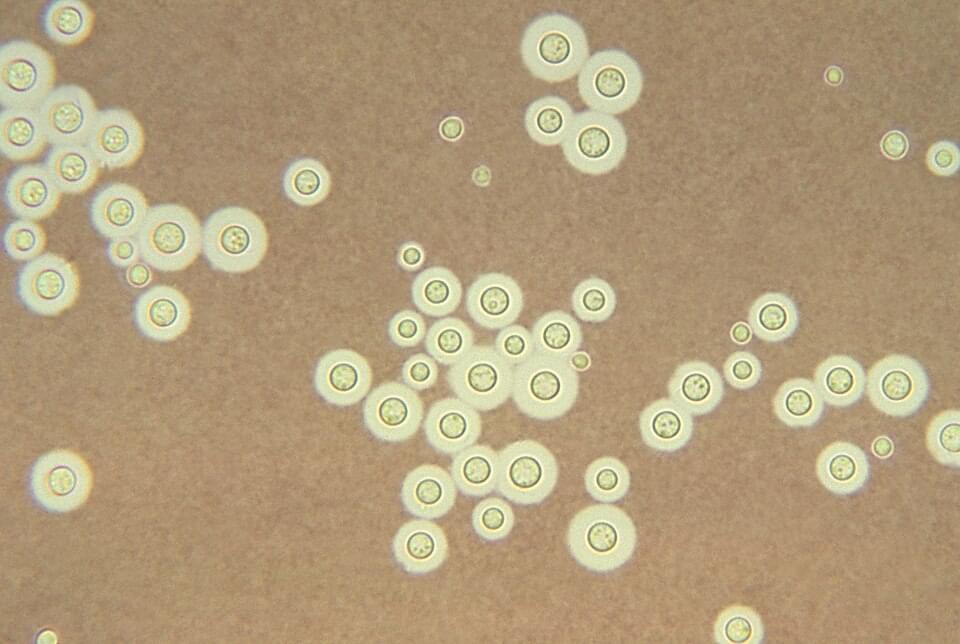
Cryptococcus neoformans, discovered at the site in 1991, feeds on radiation through a process called radiosynthesis. Its high levels of melanin absorb harmful radiation and convert it into chemical energy, much like how plants use photosynthesis to create energy.
NASA scientists, in collaboration with Johns Hopkins University, are now testing melanin extracted from the fungi aboard the International Space Station. ’ If successful, this natural shield could protect astronauts and equipment from cosmic rays, a significant challenge for long-term space exploration. “Space radiation is dangerous and damages matter,” explains researcher Radamés J.B. Cordero. “A material like this could shield astronauts and benefit people here on Earth.” This discovery turns a remnant of a nuclear disaster into a potential lifesaver for humanity’s journey into the cosmos.
Learn more.
Radiotrophic fungi are fungi that can perform the hypothetical biological process called radiosynthesis, which means using ionizing radiation as an energy source to drive metabolism. It has been claimed that radiotrophic fungi have been found in extreme environments such as in the Chernobyl Nuclear Power Plant.
Most radiotrophic fungi use melanin in some capacity to survive. [ 1 ] The process of using radiation and melanin for energy has been termed radiosynthesis, and is thought to be analogous to anaerobic respiration. [ 2 ] However, it is not known if multi-step processes such as photosynthesis or chemosynthesis are used in radiosynthesis or even if radiosynthesis exists in living organisms.