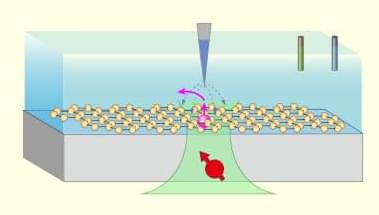
For the first time, researchers at the Technical University of Munich (TUM) have succeeded in using nanorobots to stimulate stem cells with such precision that they are reliably transformed into bone cells. To achieve this, the robots exert external pressure on specific points in the cell wall. The new method offers opportunities for faster treatments in the future.
Prof. Berna Özkale Edelmann’s nanorobots consist of tiny gold rods and plastic chains. Several million of them are contained in a gel cushion measuring just 60 micrometers, together with a few human stem cells. Powered and controlled by laser light, the robots, which look like tiny balls, mechanically stimulate the cells by exerting pressure.
“We heat the gel locally and use our system to precisely determine the forces with which the nanorobots press on the cell—thereby stimulating it,” explains the professor of nano-and microrobotics at TUM. This mechanical stimulation triggers biochemical processes in the cell. Ion channels change their properties, and proteins are activated, including one that is particularly important for bone formation.