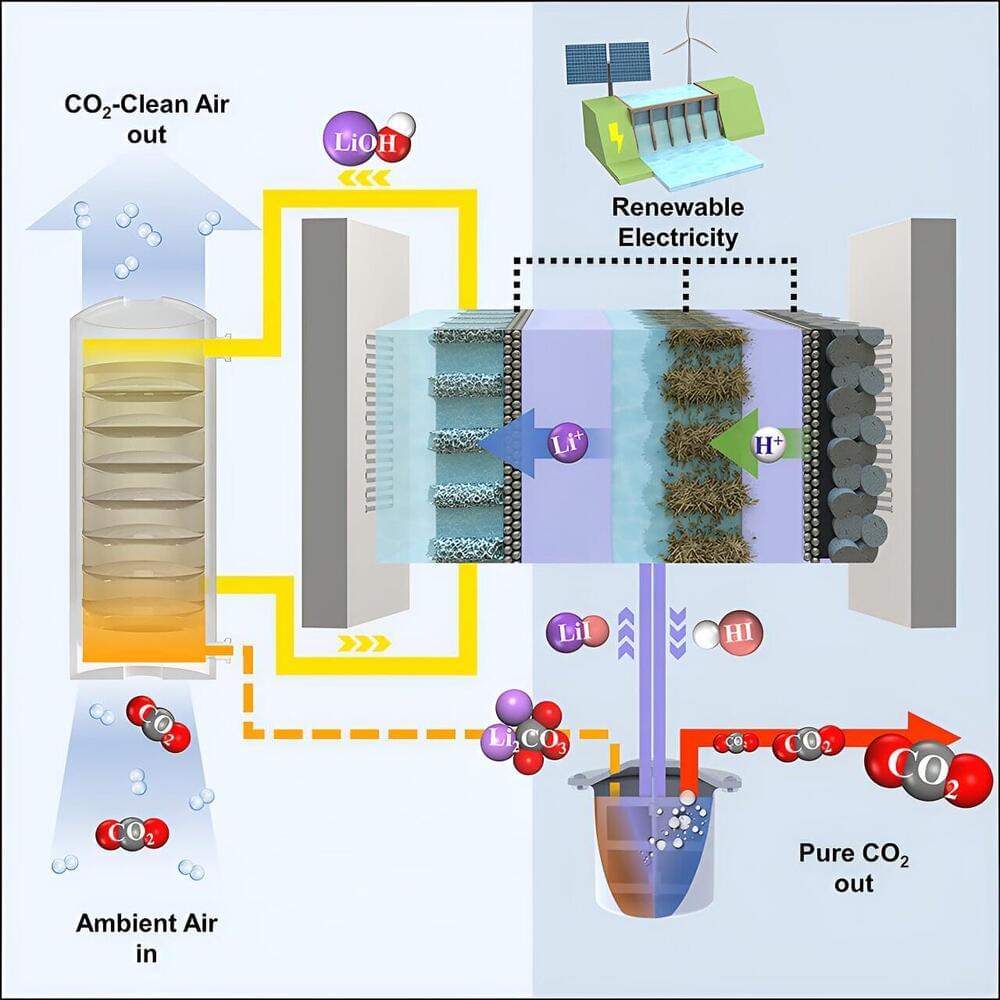
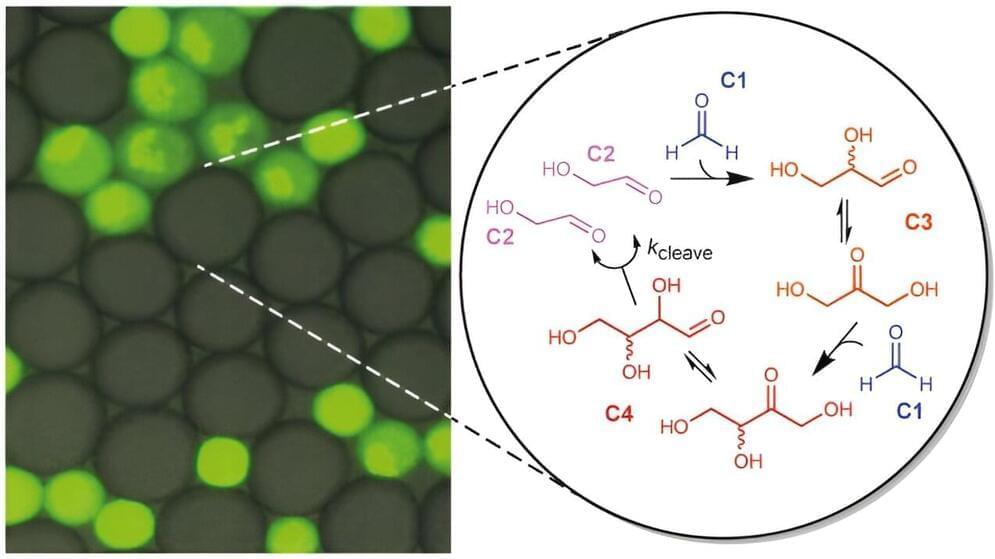
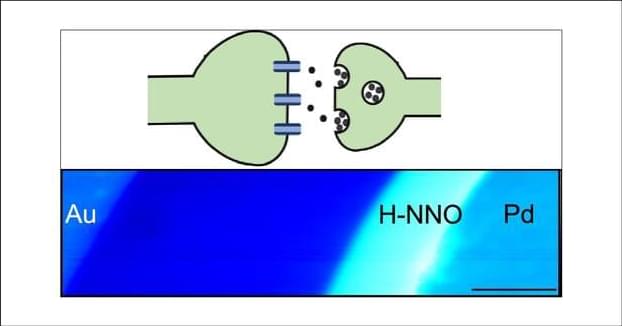
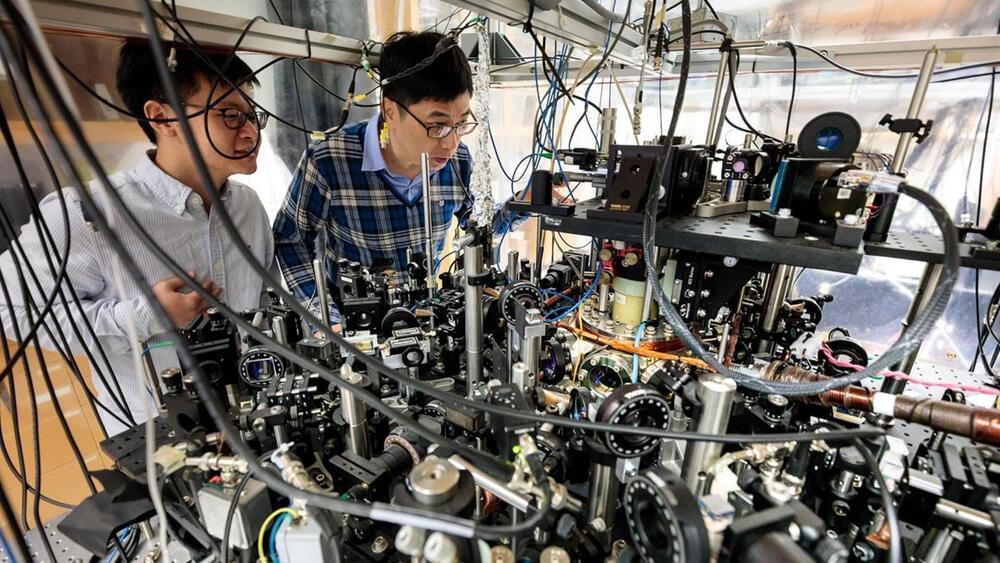
A team from the University of Toronto’s Faculty of Applied Science & Engineering has invented a device that leverages electrochemistry to increase the efficiency of direct air carbon capture. Their alternative strategy aims to accelerate the widespread adoption of this emerging technology.
“The technology required to pull carbon directly out of the air has been developing for decades, but the field is now accelerating with governments and industry investing in the infrastructure required to actually do this at scale,” says David Sinton, a professor in the faculty’s department of mechanical and industrial engineering and senior author on a paper published in Joule that outlines the new technique.
“One key barrier is that current processes require a lot of energy, and indeed emit a fair amount of carbon themselves,” says Sinton, who holds a Canada Research Chair in microfluidics and energy and is academic director of the Climate Positive Energy Initiative, one of U of T’s Institutional Strategic Initiatives.