Researchers discovered dopamine plays a crucial role in adapting decisions to changing situations. Using PET and fMRI scans, they found dopamine increases in the brain’s reward center during task changes, aiding learning from mistakes.

The optic nerve has been partly regenerated in mice, raising hopes for treating blindness caused by conditions such as glaucoma.
Ultimate fact presents top 15 Weapons Of The Future Will Blow Your Mind. We’ve come a long way since sticks and stones, and it’s almost inconceivable that only a few hundred years ago, Man was still waging war with bows, arrows, cannons, and muskets. Modern militaries are constantly in the process of developing new weapons, some of which will definitely make some mouths drop. We thought it would be fun to take a closer look at the most amazing offensive and defensive weapons currently in the works.
Autonomous weapons.
These are robotic vehicles, under development, that search and destroy enemy troops and equipment on the ground or in the air, without risk to friendly troops – theoretically.
Onboard computers interpret sensor data to identify and target hostile forces with built-in weapons. Robots may query human controllers at remote sites for the go-ahead to fire, and friendly forces may carry transponders that identify them as “friends”
High-energy lasers.
These are powerful energy beams that travel through air or space in straight lines. They travel at the speed of light and can strike over distances of thousands of kilometres. Large mirrors focus powerful laser beams onto a small spot on the target.
Space-based weapons.
Space is the ultimate high ground, so weapons in orbit would have the ability to see and zap anything on the ground, in the air, or nearby in space. The main mission of space-based weapons would be to defend against ballistic missiles fired at targets on Earth.
Hypersonic aircraft.
Launched from a standard runway, a hypersonic aircraft could fly faster than Mach 5 to strike anywhere in the world within two hours. It would also have enough thrust to deliver a satellite to low-Earth orbit. To get off the ground from a runway, a hypersonic plane would either hitch a ride on a conventional plane, or have its own conventional jet engine.
Active Denial System.
Millimetre-wave or microwave beams supposedly make people flee without injuring them. They might typically be powered by a generator fitted to a Humvee, in crowd control situations.
A 2-metre antenna and mobile generator produce and aim a beam of 95-gigahertz (3-millimetre) radiation.
Nuclear missiles.
Nuclear missiles are able to deliver unmatched destructive power anywhere in the world, making them the ultimate level of military power. One or more nuclear warheads are mounted on a ballistic missile, and launched vertically.
Stun guns (Tasers)
Tasers disable people with bursts of high-voltage electricity, allowing police to subdue them without lasting injury. A special gun fires darts on wires. These deliver a pulse of electricity that temporarily disrupts control of voluntary muscles.
E-bombs.
A rapid increase in electromagnetic field strength during a pulse, induces surges of electric current in conductors. This burns out electrical equipment – semiconductor chips are particularly vulnerable.
Layered missile defence.
Layered missile defence offers the best chance to shoot down attacking ballistic missiles.
Multiple anti-missile systems are deployed to target ballistic missiles during different stages of the attacking missile’s flight: Each phase, or layer, of defence increases the chance of successful destruction of the missile.
Information warfare.
This technique interferes with the flow of information vital to enemy operations, while defending friendly channels of communication. Information warfare specifically targets communication networks and computers.
‘Hyper Stealth’ or ‘Quantum Stealth’
Using naturally occurring metamaterials, scientists have been designing lightwave-bending materials that can greatly reduce the thermal and visible signatures of a target.
Electromagnetic Rail Guns.
EM rail gun launchers use a magnetic field rather than chemical propellants (e.g., gunpowder or fuel) to thrust a projectile at long range and at velocities of 4,500 mph to 5,600 mph. nautical miles using 32 megajoules.
The extended velocity and range of EM rail guns provides several benefits both in offensive and defensive terms, from precision strikes that can counter even the most advanced area defense systems to air defense against incoming targets.
Space Weapons.
Despite international pressure against the weaponization of space, major countries continue to explore technologies that would turn the sky above us into the next battleground.
Hypersonic Cruise Missiles and ‘Prompt Global Strike’
Had hypersonic cruise missiles existed in the mid-1990s, the U.S. might have rid itself of Al Qaeda leader Osama bin Laden much earlier than it did, and would have accomplished the feat in Afghanistan rather than in Pakistan.
Sentient’ Unmanned Vehicles.
Perhaps the single-most important development in the defense industry in the past decade is the emergence of unmanned vehicles.
Among this which one seems most terrible to you let us know in the comment section.
#UltimateFact #Weaon #Facts
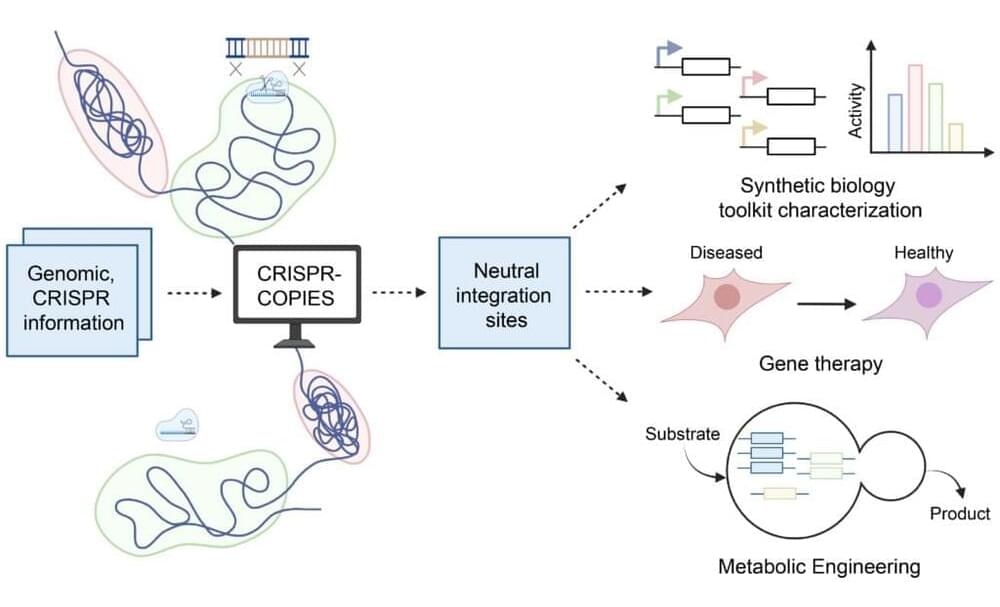
CRISPR/Cas systems have undergone tremendous advancement in the past decade. These precise genome editing tools have applications ranging from transgenic crop development to gene therapy and beyond. And with their recent development of CRISPR-COPIES, researchers at the Center for Advanced Bioenergy and Bioproducts Innovation (CABBI) are further improving CRISPR’s versatility and ease of use.
“CRISPR-COPIES is a tool that can quickly identify appropriate chromosomal integration sites for genetic engineering in any organism,” said Huimin Zhao, CABBI Conversion Theme Leader and Steven L. Miller Chair of Chemical and Biomolecular Engineering (ChBE) at the University of Illinois. “It will accelerate our work in the metabolic engineering of non-model yeasts for cost-effective production of chemicals and biofuels.”
Gene editing has revolutionized scientists’ capabilities in understanding and manipulating genetic information. This form of genetic engineering allows researchers to introduce new traits into an organism, such as resistance to pests or the ability to produce a valuable biochemical.
The color centers of diamond are the focus of an increasing number of research studies, due to their potential for developing quantum technologies. Some works have particularly explored the use of negatively-charged group-IV diamond defects, which exhibit an efficient spin-photon interface, as the nodes of quantum networks.
Researchers at Ulm University in Germany recently leveraged a Germanium vacancy (GeV) center in diamond to realize a quantum memory. The resulting quantum memory, presented in a Physical Review Letters paper, was found to exhibit a promising coherence time of more than 20 ms.
“Our research group’s primary focus is the exploration of diamond color centers for quantum applications,” Katharina Senkalla, co-author of the paper, told Phys.org. “The most popular defect of diamond so far has been the nitrogen-vacancy center, but, recently, other color centers have also become a focus of research. These consist of an element from the IV column of the periodic table—Si, Ge, Sn or Pb, and a lattice vacancy (i.e., missing next-neighbor carbon atom).”

This isn’t rocket science it’s neuroscience.
Ever since the dawn of antiquity, people have strived to improve their cognitive abilities. From the advent of the wheel to the development of artificial intelligence, technology has had a profound leverage on civilization. Cognitive enhancement or augmentation of brain functions has become a trending topic both in academic and public debates in improving physical and mental abilities. The last years have seen a plethora of suggestions for boosting cognitive functions and biochemical, physical, and behavioral strategies are being explored in the field of cognitive enhancement. Despite expansion of behavioral and biochemical approaches, various physical strategies are known to boost mental abilities in diseased and healthy individuals. Clinical applications of neuroscience technologies offer alternatives to pharmaceutical approaches and devices for diseases that have been fatal, so far. Importantly, the distinctive aspect of these technologies, which shapes their existing and anticipated participation in brain augmentations, is used to compare and contrast them. As a preview of the next two decades of progress in brain augmentation, this article presents a plausible estimation of the many neuroscience technologies, their virtues, demerits, and applications. The review also focuses on the ethical implications and challenges linked to modern neuroscientific technology. There are times when it looks as if ethics discussions are more concerned with the hypothetical than with the factual. We conclude by providing recommendations for potential future studies and development areas, taking into account future advancements in neuroscience innovation for brain enhancement, analyzing historical patterns, considering neuroethics and looking at other related forecasts.
Keywords: brain 2025, brain machine interface, deep brain stimulation, ethics, non-invasive and invasive brain stimulation.
Humans have striven to increase their mental capacities since ancient times. From symbolic language, writing and the printing press to mathematics, calculators and computers, mankind has devised and employed tools to record, store, and exchange thoughts and to enhance cognition. Revolutionary changes are occurring in the health care delivery system as a result of the accelerating speed of innovation and increased employment of technology to suit society’s evolving health care needs (Sullivan and Hagen, 2002). The aim of researchers working on cognitive enhancement is to understand the neurobiological and psychological mechanisms underlying cognitive capacities while theorists are rather interested in their social and ethical implications (Dresler et al., 2019; Oxley et al., 2021).
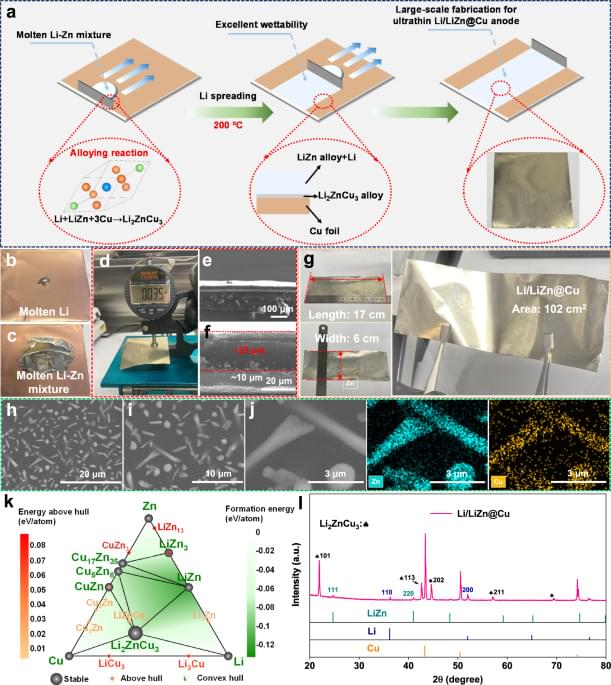
Utilizing an ultra-thin Li anode with a thickness below 50 μm is crucial for enhancing the energy density of batteries. Here, the authors develop a finely tunable, thin alloy-based Li anode that features a hierarchical Li electrochemistry, enabling stable cycling and superior energy density in Li metal batteries.

The authors of a recent review published in Ageing Research Reviews summarize the research on epigenetic reprogramming and its potential as a rejuvenation therapy [1].
Aging leads to changes in the epigenome. Those changes can lead to alterations in gene regulation, affecting cellular homeostasis, and can play a role in age-associated phenotypes. Epigenetic modifications, the addition or removal of chemical groups to the DNA or DNA-associated proteins, have a profound impact on gene expression, tissue functions, and identity [2].
This review’s authors believe epigenetic reprogramming to be among the most currently promising interventions to stop or delay aging, potentially even reversing it at the cellular level. They believe that epigenetics are the basis of aging; therefore, being able to impact the epigenome would allow them to address multiple Hallmarks of Aging simultaneously.
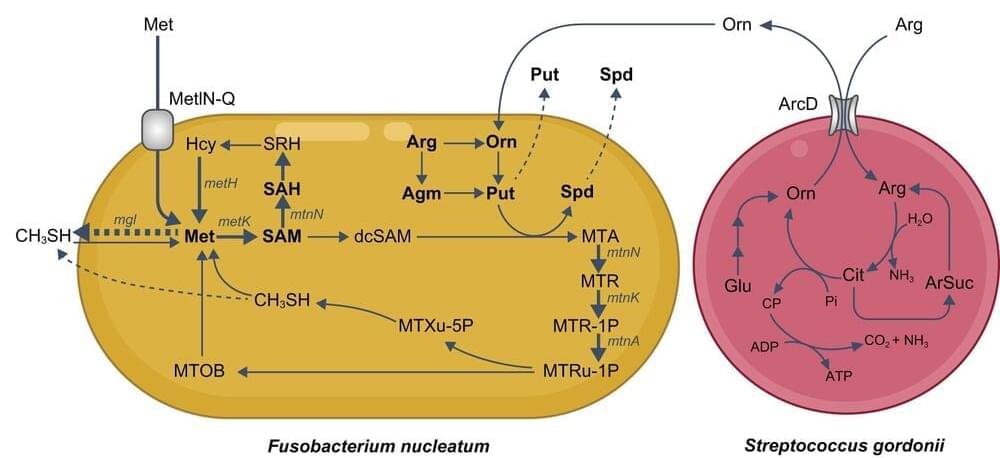
In a study published last month in mSystems, researchers from Osaka University revealed that the interaction between two common types of oral bacteria leads to the production of a chemical compound that is a major cause of smelly breath.
Bad breath is caused by volatile compounds that are produced when bacteria in the mouth digest substances like blood and food particles. One of the smelliest of these compounds is methyl mercaptan (CH3SH), which is produced by microbes that live around the teeth and on the surface of the tongue. However, little is known about which specific bacterial species are involved in this process.
“Most previous studies investigating CH3SH-producing oral bacteria have used isolated enzymes or relatively small culture volumes,” explains lead author of the study Takeshi Hara. “In this study, we aimed to create a more realistic environment in which to investigate CH3SH production by major oral bacteria.”
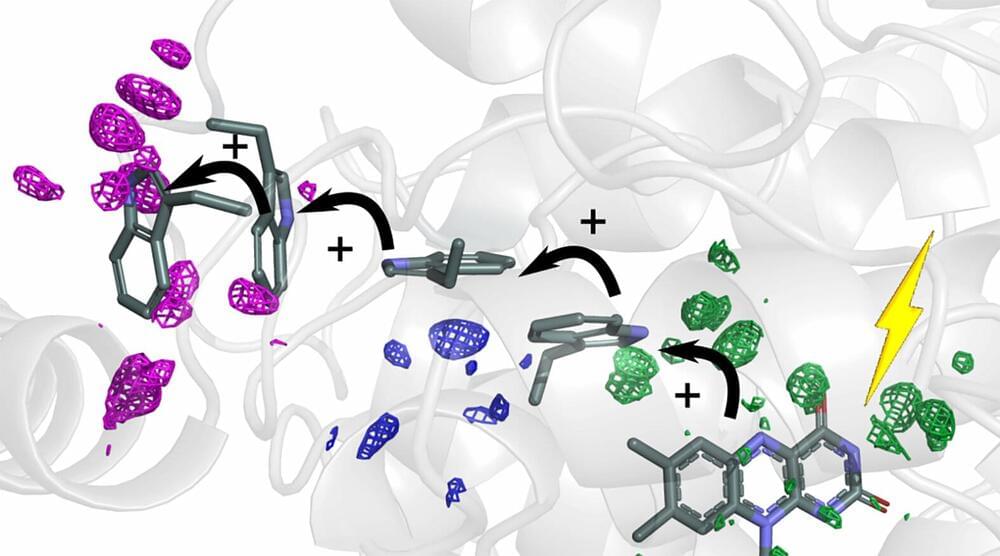
Cells need energy to function. Researchers at the University of Gothenburg can now explain how energy is guided in the cell by small atomic movements to reach its destination in the protein. Imitating these structural changes of the proteins could lead to more efficient solar cells in the future.
The sun’s rays are the basis for all the energy that creates life on Earth. Photosynthesis in plants is a prime example, where solar energy is needed for the plant to grow. Special proteins absorb the sun’s rays, and the energy is transported as electrons inside the protein, in a process called charge transfer. In a new study, researchers show how proteins deform to create efficient transport routes for the charges.
“We studied a protein, photolyase, in the fruit fly, whose function is to repair damaged DNA. The DNA repair is powered by solar energy, which is transported in the form of electrons along a chain of four tryptophans (amino acids). The interesting discovery is that the surrounding protein structure was reshaped in a very specific way to guide the electrons along the chain,” explains Sebastian Westenhoff, Professor of Biophysical Chemistry.