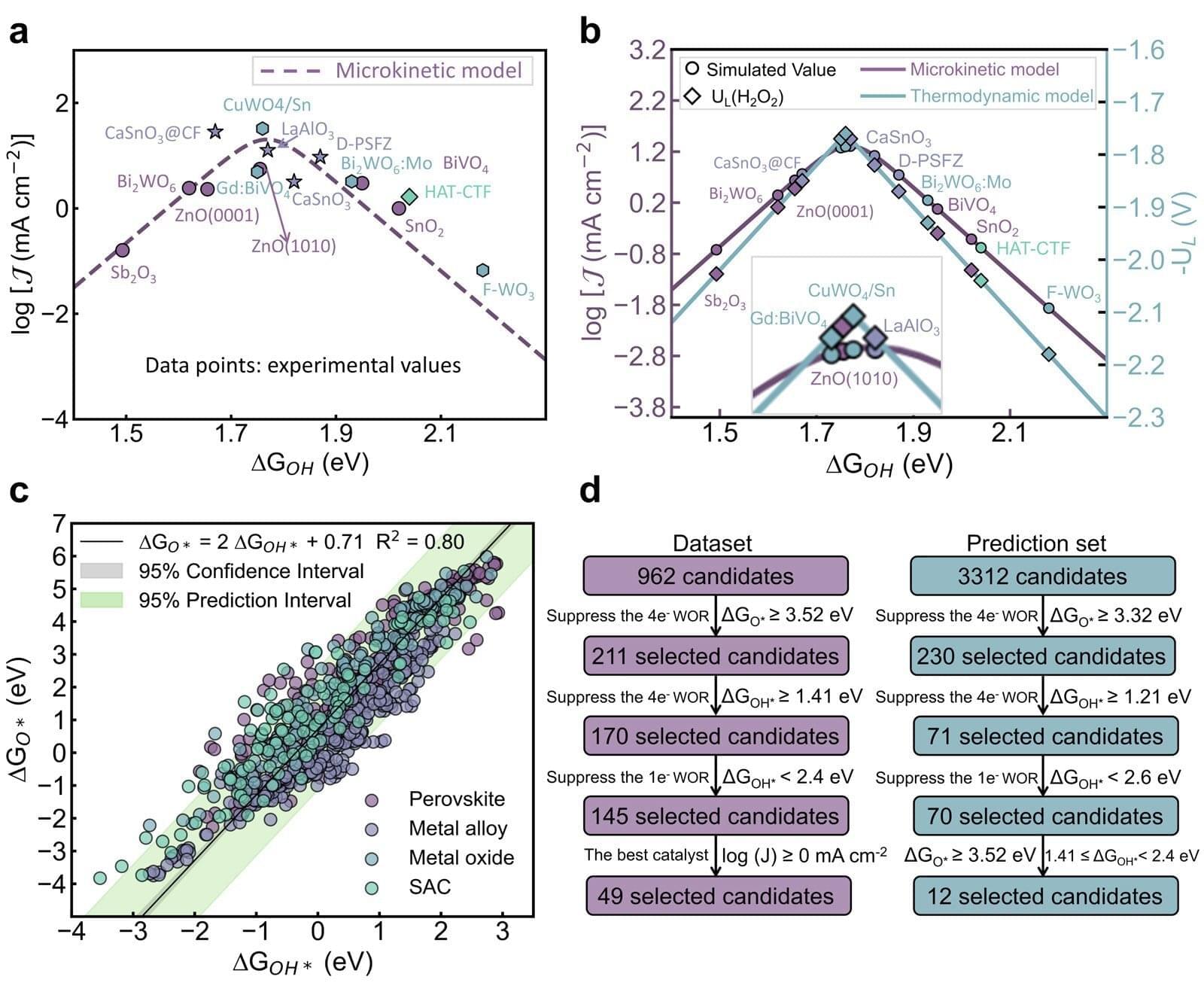
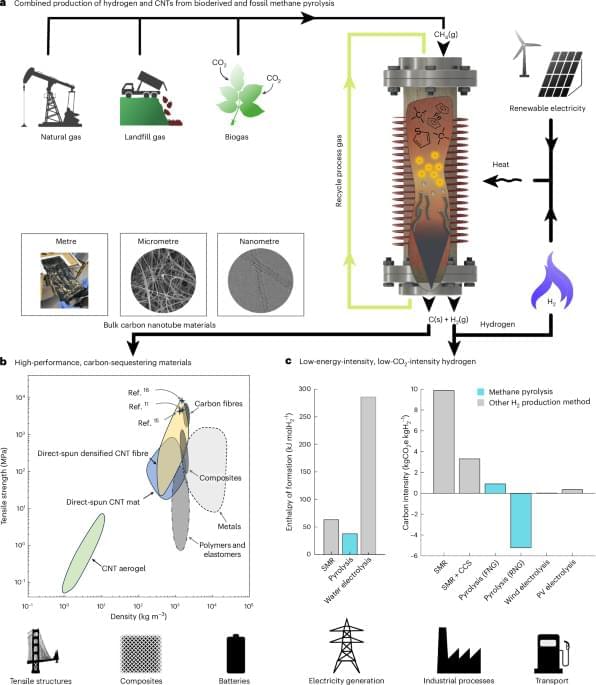
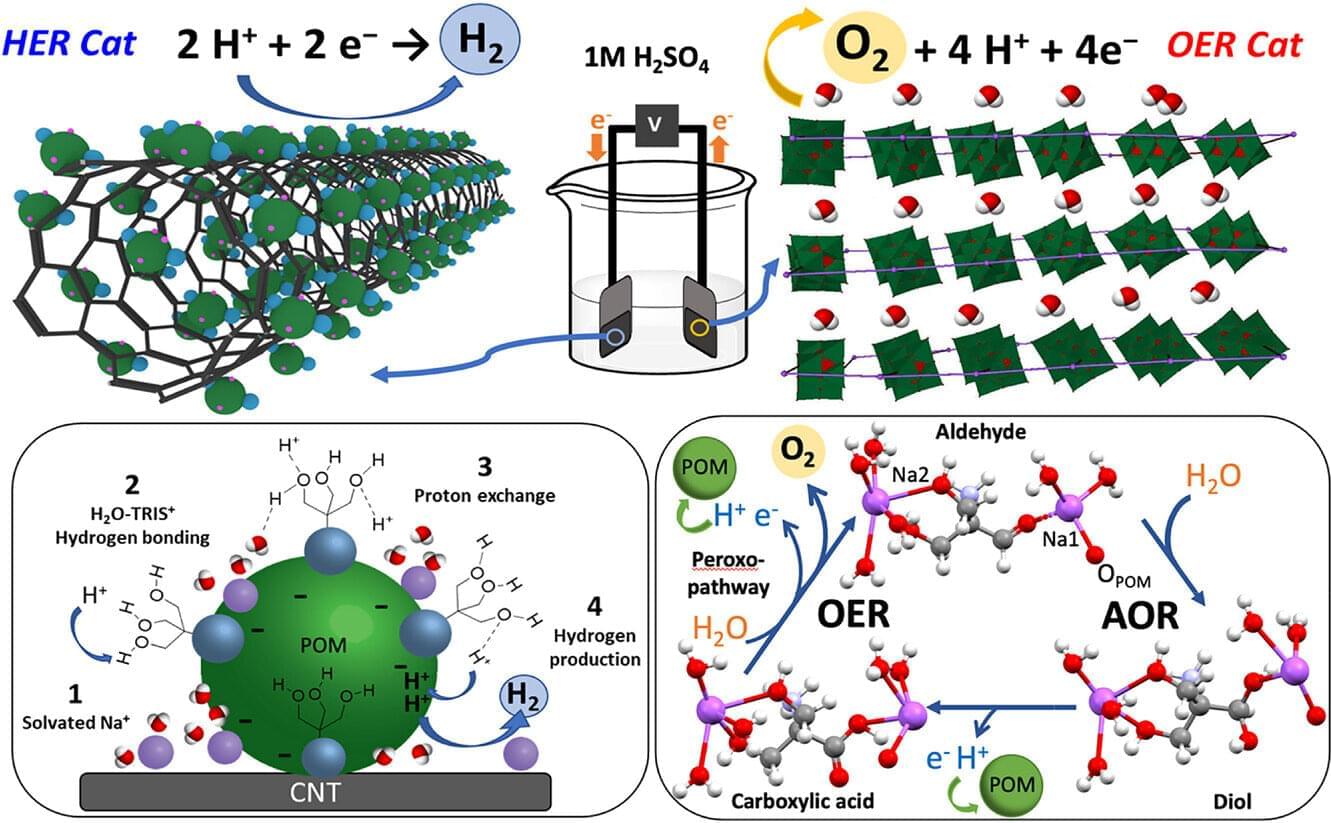
Experiments reveal that inflation not only smooths the universe but populates it with a specific distribution of initial perturbations, creating a foundation for structure formation. The team measured how quantum fluctuations during inflation are stretched and amplified, transitioning from quantum to classical behavior through a process of decoherence and coarse-graining. This process yields an emergent classical stochastic process, captured by Langevin or Fokker-Planck equations, demonstrating how classical stochastic dynamics can emerge from underlying quantum dynamics. The research highlights that the “initial conditions” for galaxy formation are not arbitrary, but constrained by the Gaussian field generated during inflation, possessing specific correlations. This framework provides a cross-scale narrative, linking microphysics and cosmology to life, brains, culture, and ultimately, artificial intelligence, demonstrating a continuous evolution of dynamics across the universe.
Universe’s Evolution, From Cosmos to Cognition
This research presents a unified, cross-scale narrative of the universe’s evolution, framing cosmology, astrophysics, biology, and artificial intelligence as successive regimes of dynamical systems. Rather than viewing these fields as separate, the work demonstrates how each builds upon the previous, connected by phase transitions, symmetry-breaking events, and attractors, ultimately tracing a continuous chain from the Big Bang to contemporary learning systems. The team illustrates how gravitational instability shapes the cosmic web, leading to star and planet formation, and how geochemical cycles establish stable, long-lived attractors, providing the foundation for life’s emergence as self-maintaining reaction networks. The study emphasizes that the universe is not simply evolving in state, but also in its capacity for description and learning, with each transition.