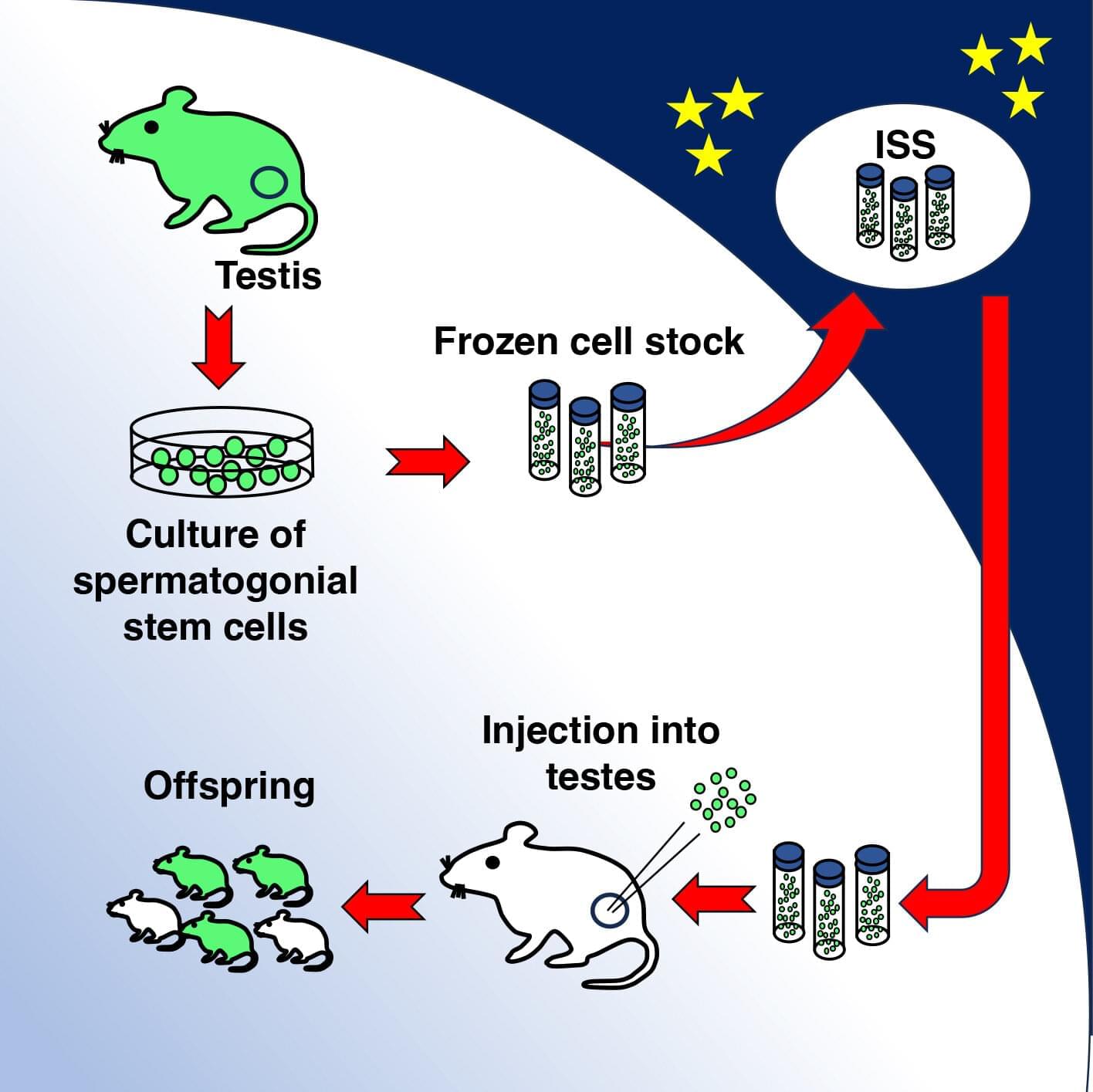
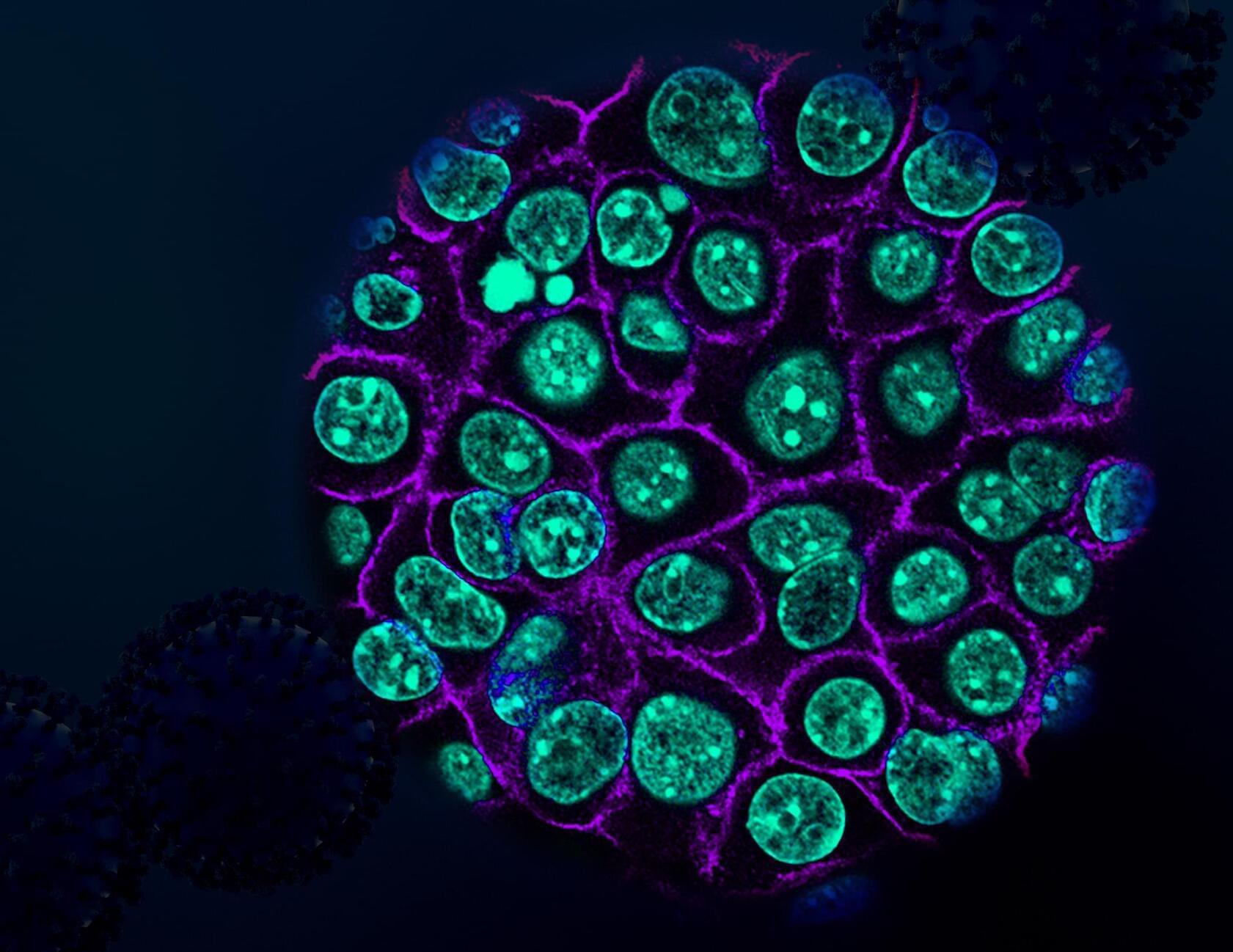
A new study, led by researchers at Children’s Hospital of Philadelphia (CHOP), identified tiny pieces of messenger RNA that are missing in pediatric high-grade glioma tumors but not in normal brain tissues. Preclinical research indicates that these missing RNA fragments can make difficult-to-treat tumors more responsive to immunotherapy. The findings were recently published in the journal Cell Reports.
One of the biggest challenges facing cancer research is the need to find safe and effective therapies for the most aggressive types of brain tumors. Adoptive immunotherapies with CAR-T cells are promising; however, they often also target healthy cells, which share most surface proteins with cancerous cells. While this collateral damage might be tolerable in patients with certain types of blood cancer, in the brain, wiping out healthy neurons is unacceptable. This means that deep knowledge of gene expression patterns exclusive to tumor cells is critical.
A potential means of discovering new therapeutic targets for brain tumors may lie in alternative splicing, a process whereby a single gene produces multiple proteins by rearranging exons, the building blocks of messenger RNA, in different combinations. Researchers suspected that splicing in glioma cells may differ from splicing in normal brain cells, which could help devise new therapeutic interventions.