New ‘cassette tape’ made of DNA has the capacity to store 36 petabytes of data, which could change the future of digital archiving.


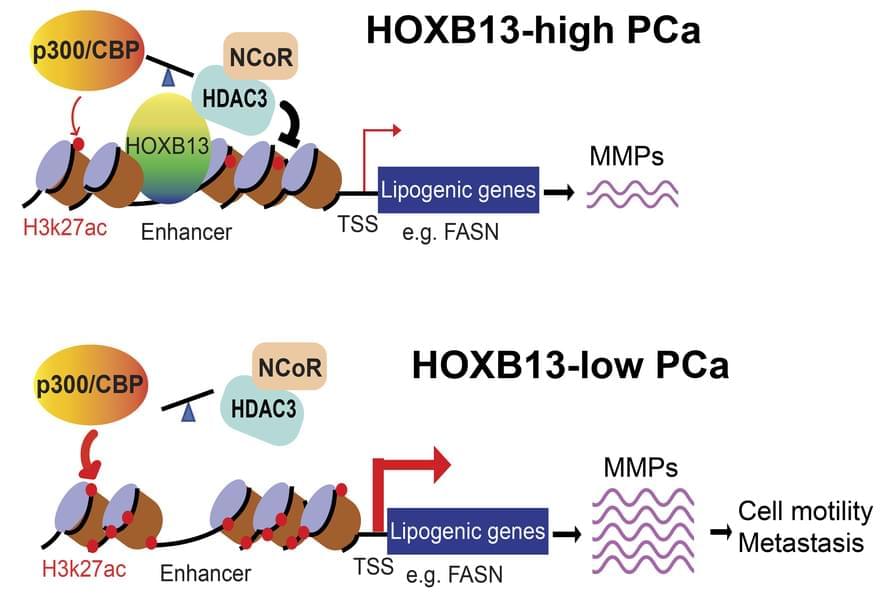

Abstract The review examines modern advances in the genetics of aging and life span. The key molecular mechanisms regulating aging processes at the genetic level are analyzed, including signaling pathways and longevity genes identified in studies on model organisms and through genome analysis of long-lived species. Special attention is given to the insulin/IGF-1 signaling pathway, the role of the FOXO transcription factor, DNA repair systems, epigenetic regulation, and modulation of mTOR and AMPK kinase activity. Results of experimental studies on increasing the life span of model organisms through genetic manipulations and combined approaches are presented.
A man has become the seventh person to be left HIV-free after receiving a stem cell transplant to treat blood cancer. Significantly, he is also the second of the seven who received stem cells that were not actually resistant to the virus, strengthening the case that HIV-resistant cells may not be necessary for an HIV cure.
“Seeing that a cure is possible without this resistance gives us more options for curing HIV,” says Christian Gaebler at the Free University of Berlin.
Image: STEVE GSCHMEISSNER/SCIENCE PHOTO LIBRARY
A handful of people with HIV have been cured after receiving HIV-resistant stem cells – but a man who received non-resistant stem cells is also now HIV-free.
By Carissa Wong

New research shows how human cells can be effectively ‘recharged’ by replacing their internal batteries – microscopic power stations called mitochondria – and the discovery could have wide-ranging benefits across healthcare and medical treatments.
The stacks of mitochondria in most of our cells naturally decline in numbers, slow down, and wear out with age. Once they start operating below peak capacity, they can contribute to multiple diseases everywhere from the heart to the brain.
In this latest study, researchers from Texas A&M University used special flower-shaped particles called nanoflowers to scavenge damaging oxygen molecules, triggering genes that increase the number of mitochondria in human stem cells.
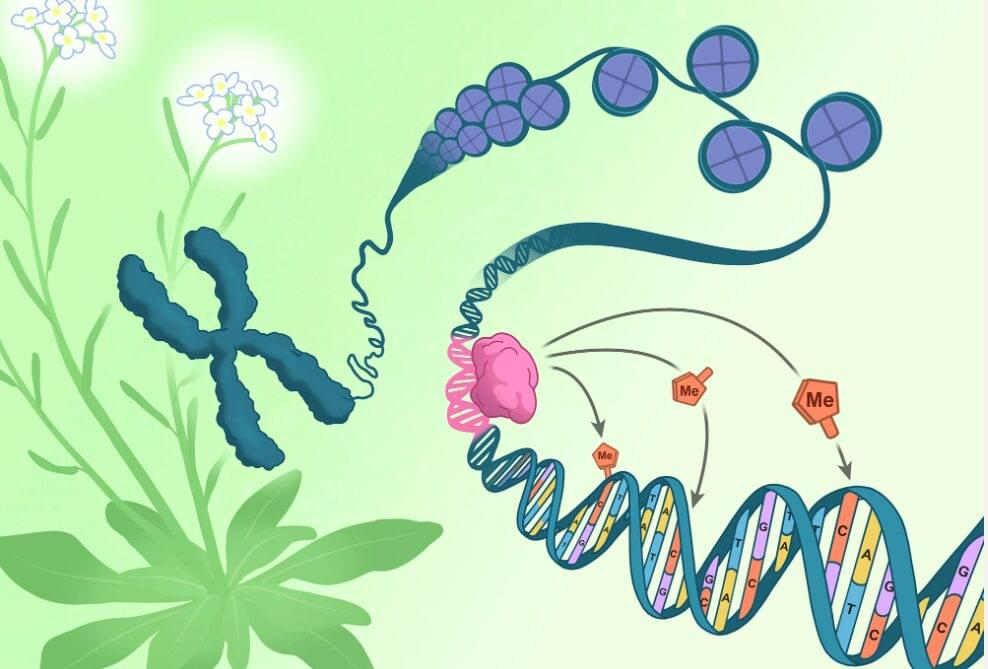
A chromosome pulled from the flowers of Arabidopsis thaliana (green and white) unspools to reveal DNA (blue) coiled around packaging-proteins called histones (purple). The direction of epigenetic changes by genetic features begins as the RIM transcription factor (pink) docks on a corresponding DNA sequence (pink). Once docked, the RIM transcription factor directs methylation machinery to tack methyl groups (orange) onto specific nearby cytosines (orange). Click here for a high-resolution image. Credit: Salk Institute.
All the cells in an organism have the exact same genetic sequence. What differs across cell types is their epigenetics—meticulously placed chemical tags that influence which genes are expressed in each cell. Mistakes or failures in epigenetic regulation can lead to severe developmental defects in plants and animals alike. This creates a puzzling question: If epigenetic changes regulate our genetics, what is regulating them?
Scientists at the Salk Institute have now used plant cells to discover that a type of epigenetic tag, called DNA methylation, can be regulated by genetic mechanisms. This new mode of plant DNA methylation targeting uses specific DNA sequences to tell the methylation machinery where to dock. Prior to this study, scientists had understood only how DNA methylation was regulated by other epigenetic features, so the discovery that genetic features can also guide DNA methylation patterns is a major paradigm shift.
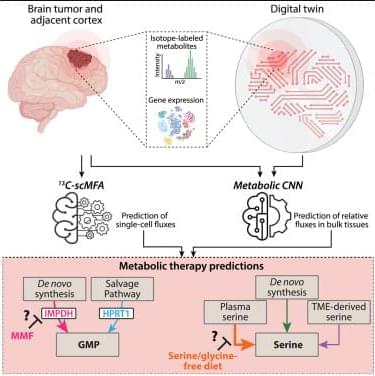
Quantifying metabolic activity in patient tumors could advance personalized cancer targeting. Meghdadi et al. develop a digital twin framework using machine learning to quantify metabolic fluxes in tissues from patients with glioma, identifying which patients may benefit from different targeted metabolic therapies like specialized diets or pharmacologic agents.
Tumor-treating fields (TTFields) are gaining traction as evidence expands beyond early enthusiasm, Medscape reports. Once considered experimental, TTFields are now supported by multiple randomized trials and are being tested across a growing list of solid tumors, positioning the therapy as a potential addition to standard cancer care in selected patients.
Here’s a look at how it works, the body of evidence, and the limitations.
Tumor treating fields use low intensity, alternating electric fields to disrupt cancer cell division.
The electric fields are generated by a wearable device — Optune Gio for glioblastoma and Optune Lua for pleural mesothelioma and NSCLC — developed and marketed by Switzerland-based oncology company Novocure.