CRISPR, the Nobel Prize-winning gene editing technology, is poised to have a profound impact on the fields of microbiology and medicine yet again.
A team led by CRISPR pioneer Jennifer Doudna and her longtime collaborator Jill Banfield has developed a clever tool to edit the genomes of bacteria-infecting viruses called bacteriophages using a rare form of CRISPR. The ability to easily engineer custom-designed phages —which has long eluded the research community —could help researchers control microbiomes without antibiotics or harsh chemicals, and treat dangerous drug-resistant infections. A paper describing the work was recently published in Nature Microbiology.
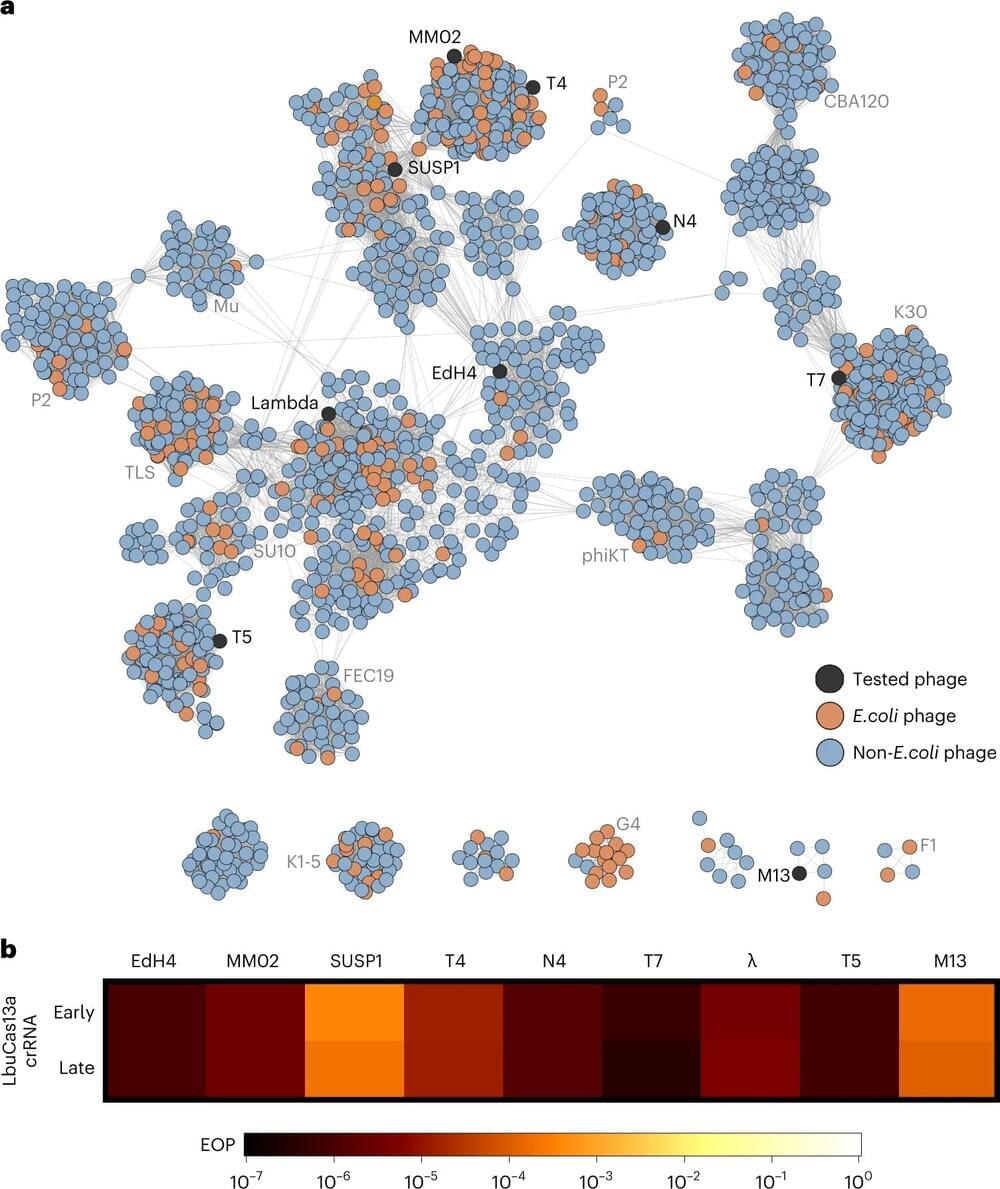
“Bacteriophages are some of the most abundant and diverse biological entities on Earth. Unlike prior approaches, this editing strategy works against the tremendous genetic diversity of bacteriophages,” said first author Benjamin Adler, a postdoctoral fellow in Doudna’s lab. “There are so many exciting directions here—discovery is literally at our fingertips.”