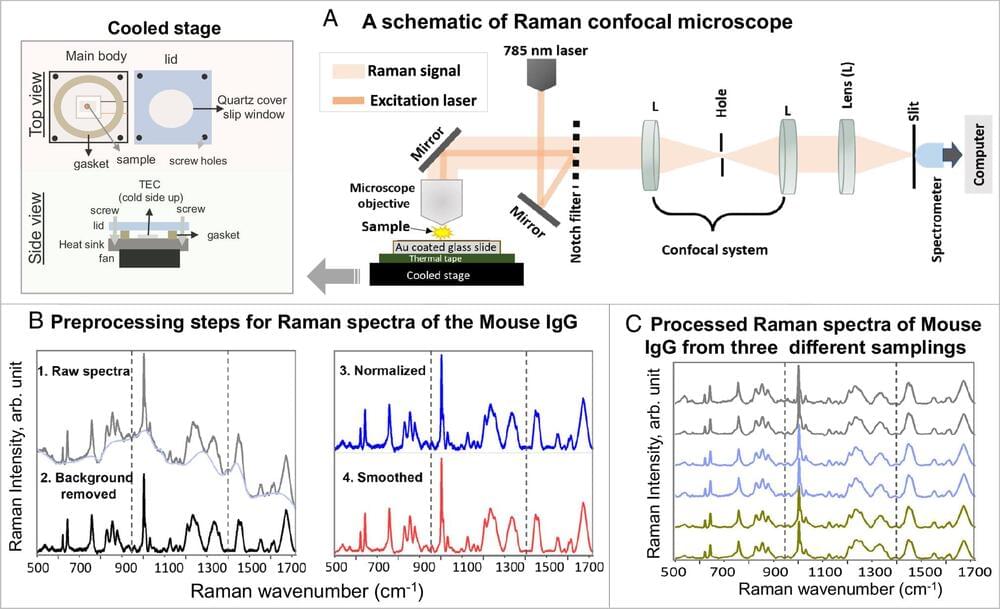
Raman spectroscopy—a chemical analysis method that shines monochromatic light onto a sample and records the scattered light that emerges—has caused frustration among biomedical researchers for more than half a century. Due to the heat generated by the light, live proteins are nearly destroyed during the optical measurements, leading to diminishing and non-reproducible results. As of recently, however, those frustrations may now be a thing of the past.
A group of researchers with the Institute for Quantum Sciences and Engineering at Texas A&M University and the Texas A&M Engineering Experiment Station (TEES) have developed a new technique that allows low-concentration and low-dose screenings of protein-to-ligand interactions in physiologically relevant conditions.
Titled thermostable-Raman-interaction-profiling (TRIP), this new approach is a paradigm-shifting answer to a long-standing problem that provides label-free, highly reproducible Raman spectroscopy measurements. The researchers published their findings in the Proceedings of the National Academy of Sciences.