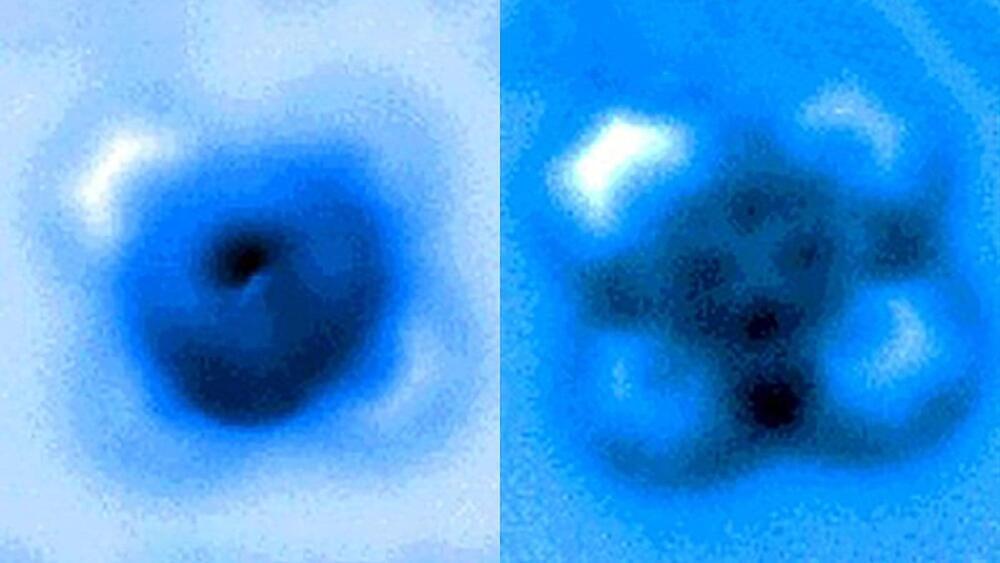
The team used a high-resolution atomic force microscope (AFM) operating in a controlled environment at Princeton’s Imaging and Analysis Center. The AFM probe, whose tip ends in a single copper atom, was moved gradually closer to the iron-carbon bond until it was ruptured. The researchers measured the mechanical forces applied at the moment of breakage, which was visible in an image captured by the microscope. A team from Princeton University, the University of Texas-Austin and ExxonMobil reported the results in a paper published Sept. 24 in Nature Communications.
“It’s an incredible image—being able to actually see a single small molecule on a surface with another one bonded to it is amazing,” said coauthor Craig Arnold, the Susan Dod Brown Professor of Mechanical and Aerospace Engineering and director of the Princeton Institute for the Science and Technology of Materials (PRISM).
“The fact that we could characterize that particular bond, both by pulling on it and pushing on it, allows us to understand a lot more about the nature of these kinds of bonds—their strength, how they interact—and this has all sorts of implications, particularly for catalysis, where you have a molecule on a surface and then something interacts with it and causes it to break apart,” said Arnold.








Comments are closed.