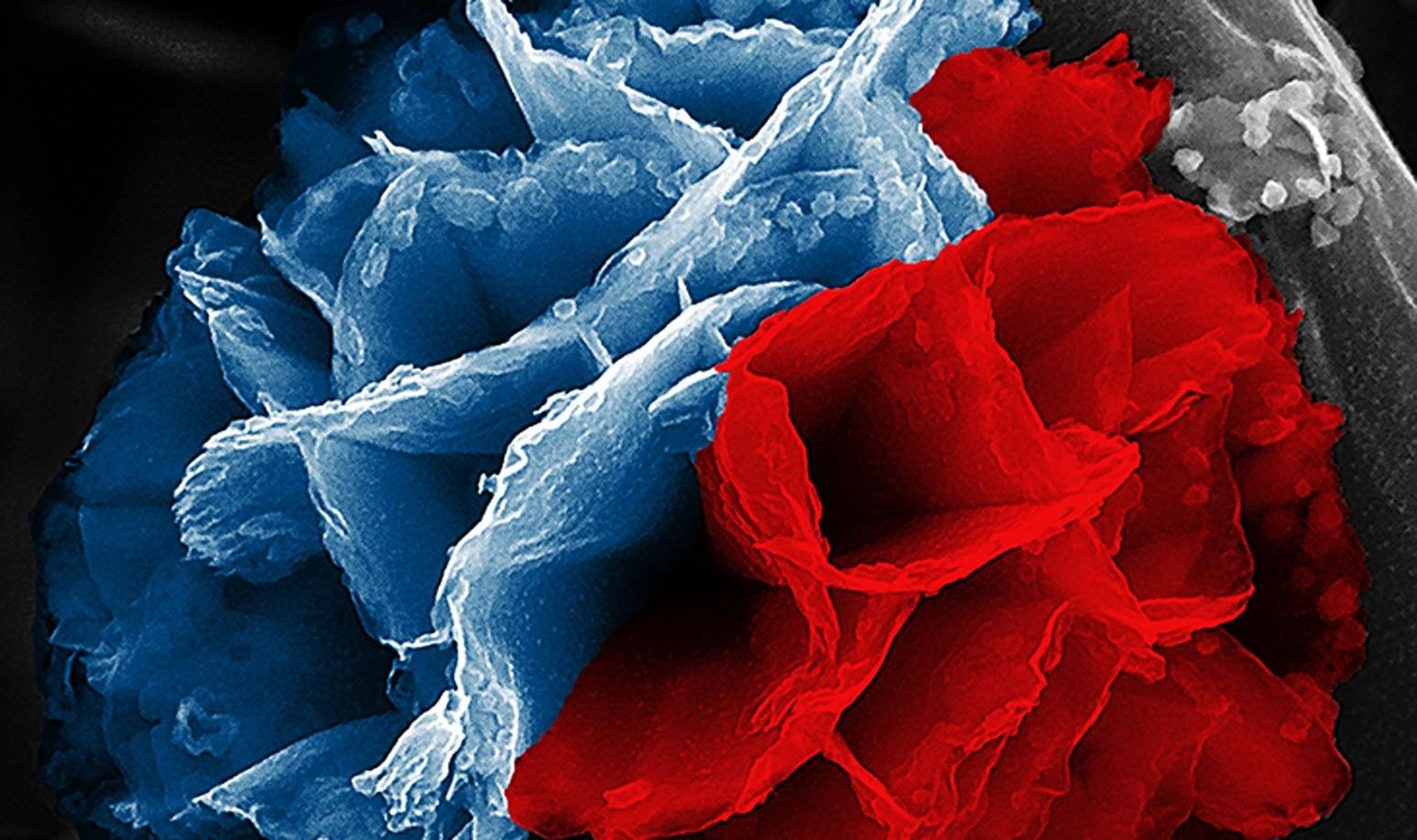
Researchers have developed a straightforward way to make a type of conducting polymers with high surface area—called “nanoflowers”—potentially useful for energy transfer and storage.
If you could brush your cheek against a nanoflower’s microscopic petals, you’d find them cool, hard, and… rusty. Common rust forms the inner skeleton of these lovely and intricate nanostructures, while their outer layer is a kind of plastic.
“Rust will always pose a challenge in Earth’s humid and oxygenated atmosphere,” says Julio M. D’Arcy, assistant professor of chemistry at Washington University in St. Louis and a member of the Institute of Materials Science and Engineering. “Corrosion makes structures fragile and decreases the ability of components to function properly. But in our lab, we’ve learned how to control the growth of rust so that it can serve an important purpose.”